ASTM E1569-03
(Test Method)Standard Test Method for Determination of Oxygen in Tantalum Powder
Standard Test Method for Determination of Oxygen in Tantalum Powder
SIGNIFICANCE AND USE
This test method is primarily intended as a test for compliance with compositional specifications. It is assumed that all who use this method will be trained analysts capable of performing common laboratory procedures skillfully and safely. It is expected that the work will be performed in a properly equipped laboratory.
SCOPE
1.1 This test method covers the determination of oxygen in tantalum powder in concentrations from 0.05 to 0.50 %.
1.2 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1569–03
Standard Test Method for
1
Determination of Oxygen in Tantalum Powder
This standard is issued under the fixed designation E 1569; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope the thermistor bridge output is integrated and processed to
display percent oxygen.
1.1 This test method covers the determination of oxygen in
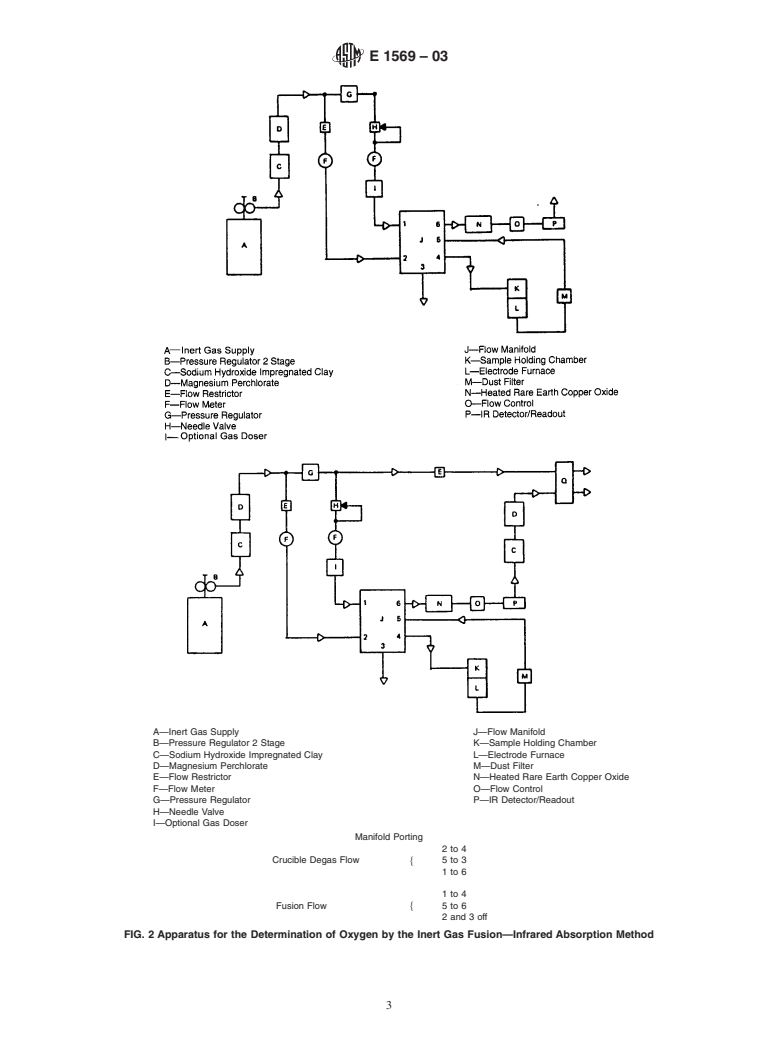
3.4 In a typical instrument based on infrared detection (see
tantalum powder in concentrations from 0.05 to 0.50 %.
Fig. 2), the sample is fused in a stream of argon and passed
1.2 This standard does not purport to address all of the
directly into an infrared cell through which infrared energy is
safety problems, if any, associated with its use. It is the
transmitted. The CO in the sample gases absorbs some of the
responsibility of the user of this standard to establish appro-
transmittedinfraredenergyandthedecreaseinenergyreaching
priate safety and health practices and determine the applica-
the detector is processed and displayed directly as percent
bility of regulatory limitations prior to use.
oxygen.
2. Referenced Documents
4. Significance and Use
2.1 ASTM Standards:
4.1 This test method is primarily intended as a test for
E 29 Practice for Using Significant Digits in Test Data to
2
compliance with compositional specifications. It is assumed
Determine Conformance with Specifications
that all who use this method will be trained analysts capable of
E 50 Practices for Apparatus, Reagents, and Safety Precau-
3
performing common laboratory procedures skillfully and
tions for Chemical Analysis of Metals
safely. It is expected that the work will be performed in a
E 691 Practice for Conducting an Interlaboratory Study to
2
properly equipped laboratory.
Determine the Precision of a Test Method
5. Interferences
3. Summary of Test Method
5.1 The elements usually present in this material do not
3.1 This test method is intended for use with automated,
interfere but there is some evidence to suggest that low-purity
commercially available inert gas fusion analyzers.
flux can act as a getter of the released oxygen.
3.2 The sample, plus flux, is fused in a graphite crucible
under a flowing inert gas stream at a temperature sufficient to
6. Apparatus
release oxygen. The released oxygen combines with carbon
6.1 Fusion and Measurement Apparatus— The general
from the crucible to form CO that is swept by the inert gas
features of the instrument used in developing this test method
stream into either an infrared or thermal conductivity detector.
are shown in Figs. 1 and 2.
The detector output is compared to that of calibration reference
6.2 Capsules—The capsules must be made of high-purity
materials and the result is displayed as percent oxygen.
tin.
3.3 In an instrument whose detection is based upon thermal
6.3 Crucibles—The crucibles must be made of high-purity
conductivity (see Fig. 1), the sample gases are passed through
graphite and be of the dimensions recommended by the
heated rare earth copper oxide that converts CO to CO . The
2
manufacturer.
water produced during fusion is absorbed onto magnesium
6.4 Flux—The foil or baskets must be made of high-purity
perchlorate and the remaining nitrogen and carbon dioxide are
nickel and in the case of the baskets, the dimensions must meet
separated chromatographically. The nitrogen elutes first and
the requirements of the automatic sample drop, if present on
can be measured (on a dual capability instrument) or disre-
the instrument.
garded. The oxygen, as CO , enters the measuring cell last and
2
6.5 Tweezers—Solvent and acid-resistant plastic.
1
7. Reagents
This test method is under the jurisdiction of ASTM Committee E01 on
Analytical Chemistry for Metals, Ores, and Related Materials and is the direct
7.1 Acetic Acid—Reagent grade.
responsibility of Subcommittee E01.06 on Ti, Zr, W, Mo, Ta, Nb, Hf.
7.2 Acetone—Residue after evaporation must be less than
Current edition approved June 10, 2003. Published August 2003. Last previous
edition approved in 1998 as E 1569–93 (1998). 0.0005 %.
2
Annual Book of ASTM Standards, Vol 14.02.
3
Annual Book of ASTM Standards, Vol 03.05.
Copyright ©ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA19428-2959, United States.
1
---------------------- Page: 1 ----------------------
E1569–03
A—Inert Gas Supply I—Sample Holding Chamber
B—Pressure Regulator J—Electrode Furnace
C—Heated Copper K—Dust Filter
D—Sodium Hydroxide Impregnated Clay L—Heated Rare Earth Copper Oxide
E—Magnesium Perchlorate M—Magnesium Perchlorate
F—Flow Control N—Silica Column
G—Flow Manifold O—Therma
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.