ASTM D5197-97
(Test Method)Standard Test Method for Determination of Formaldehyde and Other Carbonyl Compounds in Air (Active Sampler Methodology)
Standard Test Method for Determination of Formaldehyde and Other Carbonyl Compounds in Air (Active Sampler Methodology)
SCOPE
1.1 This test method covers a procedure for the determination of formaldehyde (HCHO) and other aldehydes in air. This test method is specific for formaldehyde but, with modification, fourteen other aldehydes can be detected.
1.2 This test method involves drawing air through a cartridge containing silica gel coated with 2,4-dinitrophenylhydrazine (DNPH) reagent. Aldehydes and ketones readily form a stable derivative with the DNPH reagent. The DNPH derivative is analyzed for aldehydes and ketones utilizing high performance liquid chromatography (HPLC). The sampling procedure is a modification of U.S. EPA Method TO-11 (see 2.2).
1.3 This test method is based on the specific reaction of carbonyl compounds (aldehydes and ketones) with DNPH in the presence of an acid to form stable derivatives according to the reaction shown in Fig. 1, (where: both and are alkyl or aromatic groups (ketones), or both, or either or is a hydrogen atom (aldehydes)). The determination of formaldehyde, as a DNPH derivative, is similar to that of U.S. EPA Method TO-11 in that it utilizes HPLC. The detection limits have been extended to other aldehydes and ketones that can be determined as outlined in Section 13. This test method is suitable for determination of formaldehyde in the concentration range of high ppb to low ppm (v/v).
1.4 The sampling method gives a time-weighted average (TWA) sample. It can be used for long-term (1 to 24 h) or short-term (5 to 60 min) sampling of indoor air for formaldehyde.
1.5 The sampling flow rate, as described in this test method is presently limited to about 1.5 L/min. This limitation is principally due to the high pressure drop (>8 kPa at 1.0 L/min) across the DNPH-coated silica gel cartridges. This procedure is not compatible with pumps used in personal sampling equipment, that are inadequate for this application.
1.6 This test method instructs the user to purchase Sep-PAK chromatographic grade silica gel cartridges and apply acidified DNPH in situ to each cartridge, if the user desires to prepare his own cartridges. The silica gel in the Sep-PAK cartridge have a particle size of 55 to 105 [mu]m. Pre-coated DNPH silica gel cartridges are also available, if the user desires to purchase them.
1.7 The values stated in SI units are to be regarded as the standard.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 5197 – 97
Standard Test Method for
Determination of Formaldehyde and Other Carbonyl
Compounds in Air (Active Sampler Methodology)
This standard is issued under the fixed designation D 5197; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope graphic grade silica gel cartridges by the application of
acidified DNPH to each cartridge.
1.1 This test method covers a procedure for the determina-
1.6 The sampling flow rate, as described in this test method,
tion of formaldehyde (HCHO) and other carbonyl compounds
has been validated for sampling rates up to 1.5 L/min. This
(aldehydes and ketones) in air. This test method is most specific
flow rate limitation is principally due to the high pressure drop
for formaldehyde but, with modification, at least 13 other
(>8 kPa at 1.0 L/min) across the user prepared silica gel
carbonyl compounds can be detected and quantified.
cartridges which have a particle size of 55 to 105 μm. These
1.2 This test method involves drawing air through a car-
cartridges are not generally compatible with battery-powered
tridge containing silica gel coated with 2,4-
pumps used in personal sampling equipment (for example,
dinitrophenylhydrazine (DNPH) reagent. Carbonyl compounds
those used by industrial hygienists.
readily form stable derivatives with the DNPH reagent. The
1.7 Alternatively, pre-coated DNPH silica gel cartridges are
DNPH derivatives are analyzed for parent aldehydes and
also available and may be substituted provided they can be
ketones utilizing high performance liquid chromatography
demonstrated to perform equivalently. Some of these use
(HPLC). The sampling procedure is a modification of U.S.
silica gel of a larger particle size that results in a lower pressure
EPA Method TO-11 (see 2.2).
drop across the cartridge. These low pressure drop cartridges
1.3 This test method is based on the specific reaction of
may be more suitable for sampling air using battery-powered
carbonyl compounds with DNPH in the presence of an acid to
personal sampling pumps.
form stable derivatives according to the reaction shown in Fig.
1.8 The values stated in SI units are to be regarded as the
1, (where: both R and R are alkyl or aromatic groups
standard.
(ketones), or both, or either R or R is a hydrogen atom
1.9 This standard does not purport to address all of the
(aldehydes)). The determination of formaldehyde, as a DNPH
safety concerns, if any, associated with its use. It is the
derivative, is similar to that of U.S. EPA Method TO-11 in that
responsibility of the user of this standard to establish appro-
it utilizes HPLC with UV detection as the analytical finish. The
priate safety and health practices and determine the applica-
detection limits have been extended to other carbonyl com-
bility of regulatory limitations prior to use.
pounds that can be determined as outlined in Section 13. This
test method is suitable for determination of formaldehyde and
2. Referenced Documents
other carbonyl compounds in the concentration range from
2.1 ASTM Standards:
approximately 10 ppb to 1 ppm (v/v).
D 1193 Specification for Reagent Water
1.4 The sampling method gives a time-weighted average
D 1356 Terminology Relating to Sampling and Analysis of
(TWA) sample. It can be used for long-term (1 to 24 h) or
Atmospheres
short-term (5 to 60 min) sampling of air for formaldehyde.
D 3195 Practice for Rotameter Calibration
1.5 This test method instructs the user on how to prepare
D 3631 Test Methods for Measuring Surface Atmospheric
sampling cartridges from commercially available chromato-
Pressure
1 2
This test method is under the jurisdiction of ASTM Committee D-22 on The cartridge used in the development and performance evaluation of this test
Sampling and Analysis of Atmospheres and is the direct responsibility of Subcom- method was the Sep-Pak Plus Silica cartridge. The sole source of supply of the
mittee D22.05 on Indoor Air. cartridge known to the committee at this time is Waters Associates, 34 Maple Street,
Current edition approved March 10, 1997. Published May 1997. Originally Milford, MA 01757. If you are aware of alternative suppliers, please provide this
published as D 5197 – 91. Last previous edition D 5197 – 92. information to ASTM Headquarters. Your comments will receive careful consider-
ation at a meeting of the responsible technical committee, which you may attend.
Tejada, S. B., “Evaluation of Silica Gel Cartridges Coated in situ with Acidified
2,4-Dinitrophenylhydrazine for Sampling Aldehydes and Ketones in Air,” Interna-
tional Journal of Environmental Analytical Chemistry, Vol 26, 1986, pp. 167–185.
Annual Book of ASTM Standards, Vol 11.01.
Annual Book of ASTM Standards, Vol 11.03.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5197
4.2 After sampling, the sample cartridges are individually
capped and placed in individual bottles or other sealable
containers. Sample identifying tags or labels are attached and
the individual sample containers are then placed in a friction-
top can or other suitable sealable secondary container with a
pouch of charcoal for transport to the laboratory for analysis.
The cartridges are placed in cold storage until analysis.
Alternatively, the cartridges may be desorbed, diluted to a
known volume, and refrigerated until analysis.
NOTE 1—A heat-sealable foil-lined plastic pouch of the type included
with some commercial pre-coated DNPH cartridges may be used for
storing a DNPH-coated cartridge after sampling, if appropriate.
4.3 The DNPH-formaldehyde derivative is determined us-
ing isocratic reverse phase HPLC, equipped with an ultraviolet
(UV) absorption detector operated at 360 nm.
FIG. 1 Reaction of Carbonyl Compounds
4.4 A blank cartridge is likewise desorbed and analyzed in
D 3686 Practice for Sampling Atmospheres to Collect Or- accordance with 4.3.
ganic Compound Vapors (Activated Charcoal Tube Ad-
4.5 Formaldehyde and other carbonyl compounds in the
sorption Method)
sample are identified and quantified by comparison of their
E 177 Practice for Use of the Terms Precision and Bias in
retention times and peak heights or peak areas with those of
ASTM Test Methods
standard solutions.
E 682 Practice for Liquid Chromatography Terms and Re-
lationships
5. Significance and Use
2.2 EPA Methods:
5.1 This test method provides an analytical procedure for
Method TO-11, EPA-600/4-89-017, Compendium of Meth-
measuring formaldehyde and other carbonyl compounds in
ods for the Determination of Toxic Organic Compounds in
indoor or outdoor air.
Ambient Air, U.S. Environmental Protection Agency,
Research Triangle Park, NC, June 1988 (PB90-116 989/
6. Interferences
AS)
EPA-600/4-77-027a, Quality Assurance Handbook for Air
6.1 The solid sorbent sampling procedure is specific for
Pollution Measurement Systems, Volume II—Ambient sampling and analysis of formaldehyde. Interferences in this
Air Specific Methods, U.S. Environmental Protection
test method are certain isomeric aldehydes or ketones that may
Agency, Research Triangle Park, NC, July 1979 be unresolved by the HPLC system when analyzing for other
EPA-600/4-83-027, Technical Assistance Document for
aldehydes and ketones. Organic compounds that have the same
Sampling and Analysis of Toxic Organic Compounds in retention time and significant absorbance at 360 nm as the
Ambient Air, U.S. Environmental Protection Agency,
DNPH derivative of formaldehyde will interfere. Such inter-
Research Triangle Park, NC, June 1983 (PB90-187 014/ ferences can often be overcome by altering the separation
AS)
conditions (for example, using alternative HPLC columns or
mobile phase compositions).
3. Terminology
6.2 Formaldehyde contamination of the DNPH reagent is a
3.1 Definitions:
frequently encountered problem. The DNPH must be purified
3.1.1 For definitions of terms used in this test method, refer
by multiple recrystallizations in UV-grade acetonitrile. Recrys-
to Terminology D 1356 and Practice E 682.
tallization is accomplished, at 40 to 60°C, by slow evaporation
3.2 Definitions of Terms Specific to This Standard:
of the solvent to maximize crystal size. The purified DNPH
3.2.1 All other pertinent abbreviations and symbols are
crystals are stored under UV-grade acetonitrile until use.
defined when first cited in this test method.
Impurity levels of carbonyl compounds in the DNPH are
determined prior to use by HPLC and should be less than 0.15
4. Summary of Test Method
μg per cartridge.
4.1 A known volume of indoor air is drawn through a
6.3 Exposure of the DNPH-coated sampling cartridges to
prepacked silica gel cartridge coated with acidified DNPH, at a
direct sunlight may produce artifacts and should be avoided.
sampling rate of 0.5 to 1.2 L/min for an appropriate period of
6.4 Ozone at high concentrations has been shown to inter-
time. Both sampling rate and time are dependent upon carbonyl
fere negatively by reacting with both the DNPH and its
concentrations in the test atmosphere.
Annual Book of ASTM Standards, Vol 14.02.
7 9
Annual Book of ASTM Standards, Vol 14.01. Grosjean, D., “Ambient Levels of Formaldehyde, Acetaldehyde, and Formic
Available from the U.S. Department of Commerce, National Technical Infor- Acid in Southern California: Results of a One-Year Base-Line Study,” Environmen-
mation Service, 5285 Port Royal Rd., Springfield, VA 22161. tal Science & Technology, Vol 25, 1991, pp. 710–715.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5197
carbonyl derivatives (hydrazones) in the cartridge. The extent pre-coated DNPH cartridges.
of interference depends on the temporal variations of both the 6.5 This test method is not intended to be used for accurate
ozone and the carbonyl compounds and the duration of quantitation of acrolein in air. Inaccurate results for acrolein
sampling. Significant negative interference from ozone was may result from the formation of multiple derivative peaks, the
observed even at concentrations of formaldehyde and ozone instability of the peak ratios, or interferences from acetone and
11 12
typical of clean ambient air (2 and 40 ppbv, respectively). acetaldehyde, or combination thereof.
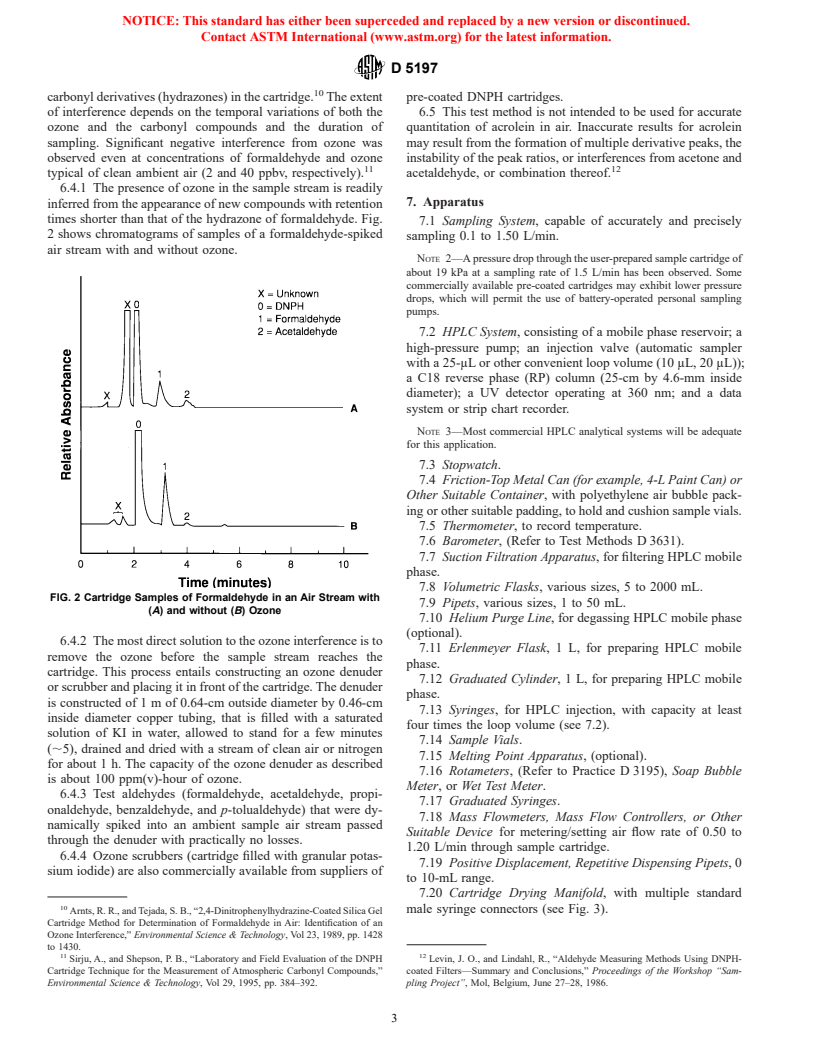
6.4.1 The presence of ozone in the sample stream is readily
7. Apparatus
inferred from the appearance of new compounds with retention
times shorter than that of the hydrazone of formaldehyde. Fig.
7.1 Sampling System, capable of accurately and precisely
2 shows chromatograms of samples of a formaldehyde-spiked
sampling 0.1 to 1.50 L/min.
air stream with and without ozone.
NOTE 2—A pressure drop through the user-prepared sample cartridge of
about 19 kPa at a sampling rate of 1.5 L/min has been observed. Some
commercially available pre-coated cartridges may exhibit lower pressure
drops, which will permit the use of battery-operated personal sampling
pumps.
7.2 HPLC System, consisting of a mobile phase reservoir; a
high-pressure pump; an injection valve (automatic sampler
with a 25-μL or other convenient loop volume (10 μL, 20 μL));
a C18 reverse phase (RP) column (25-cm by 4.6-mm inside
diameter); a UV detector operating at 360 nm; and a data
system or strip chart recorder.
NOTE 3—Most commercial HPLC analytical systems will be adequate
for this application.
7.3 Stopwatch.
7.4 Friction-Top Metal Can (for example, 4-L Paint Can) or
Other Suitable Container, with polyethylene air bubble pack-
ing or other suitable padding, to hold and cushion sample vials.
7.5 Thermometer, to record temperature.
7.6 Barometer, (Refer to Test Methods D 3631).
7.7 Suction Filtration Apparatus, for filtering HPLC mobile
phase.
7.8 Volumetric Flasks, various sizes, 5 to 2000 mL.
FIG. 2 Cartridge Samples of Formaldehyde in an Air Stream with
7.9 Pipets, various sizes, 1 to 50 mL.
(A) and without (B) Ozone
7.10 Helium Purge Line, for degassing HPLC mobile phase
(optional).
6.4.2 The most direct solution to the ozone interference is to
7.11 Erlenmeyer Flask, 1 L, for preparing HPLC mobile
remove the ozone before the sample stream reaches the
phase.
cartridge. This process entails constructing an ozone denuder
7.12 Graduated Cylinder, 1 L, for preparing HPLC mobile
or scrubber and placing it in front of the cartridge. The denuder
phase.
is constructed of1mof 0.64-cm outside diameter by 0.46-cm
7.13 Syringes, for HPLC injection, with capacity at least
inside diameter copper tubing, that is filled with a saturated
four times the loop volume (see 7.2).
solution of KI in water, allowed to stand for a few minutes
7.14 Sample Vials.
(;5), drained and dried with a stream of clean air or nitrogen
7.15 Melting Point Apparatus, (optional).
for about 1 h. The capacity of the ozone denuder as described
7.16 Rotameters, (Refer to Practice D 3195), Soap Bubble
is about 100 ppm(v)-hour of ozone.
Meter,or Wet Test Meter.
6.4.3 Test aldehydes (formaldehyde, acetaldehyde, propi-
7.17 Graduated Syringes.
onaldehyde, benzaldehyde, and p-tolualdehyde) that were dy-
7.18 Mass Flowmeters, Mass Flow Controllers, or Other
namically spiked into an ambient sample air stream passed
Suitable Device for metering/setting air flow rate of 0.50 to
through the denuder with practically no losses.
1.20 L/min through sample cartridge.
6.4.4 Ozone scrubbers (cartridge filled with granular potas-
7.19 Positive Displacement, Repetitive Dispensing Pipets,0
sium iodide) are also commercially available from suppliers of
to 10-mL range.
7.20 Cartridge Drying Manifold, with multiple standard
male syringe connectors (see Fig. 3).
Arnts, R. R., and Tejada, S. B., “2,4-Dinitrophenylhydrazine-Coated Silica Gel
Cartridge Method for Determination of Formaldehyde in Air: Identification of an
Ozone Interference,” Environmental Science & Technology, Vol 23, 1989, pp. 1428
to 1430.
11 12
Sirju, A., and Shepson, P. B., “Laboratory and Field Evaluation of the DNPH Levin, J. O., and Lindahl, R., “Aldehyde Measuring Methods Using DNPH-
Cartridge Technique for the Measurement of Atmospheric Carbonyl Compounds,” coated Filters—Summary and Conclusions,” Proceedings of the Workshop “Sam-
Environmental Science & Technology, Vol 29, 1995, pp. 384–392. pling Project”, Mol, Belgium, June 27–28, 1986.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.