ASTM F3224-17
(Test Method)Standard Test Method for Evaluating Growth of Engineered Cartilage Tissue using Magnetic Resonance Imaging
Standard Test Method for Evaluating Growth of Engineered Cartilage Tissue using Magnetic Resonance Imaging
SIGNIFICANCE AND USE
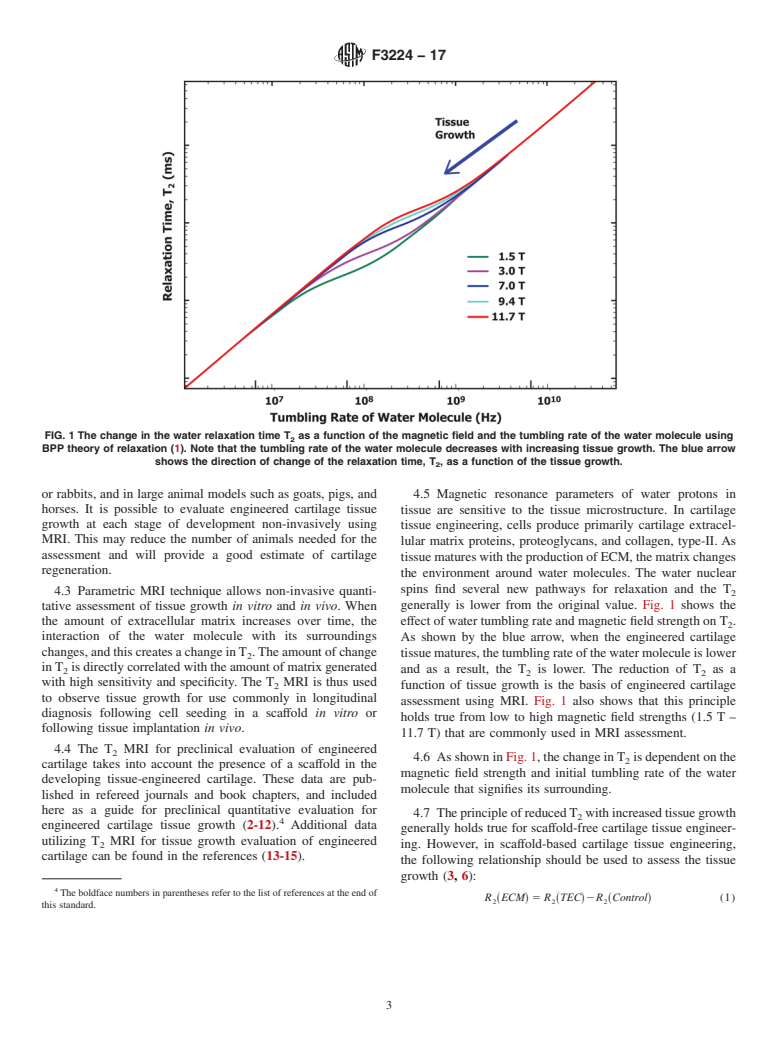
4.1 Tissue-engineered cartilage is prepared by seeding stem cells or chondrocytes in a three-dimensional biodegradable scaffold under controlled growth conditions. It is expected that the cells will differentiate towards chondrogenic lineage and produce an ample amount of cartilage extracellular matrix proteins, proteoglycans, and collagen type-II. Longitudinal assessment is needed weekly for the first few weeks in vitro and monthly at a later stage in vivo to determine the growth rate of tissue-engineered cartilage. Traditional testing methods such as histological staining, mechanical testing, and qPCR are invasive, destructive, and cannot be performed in vivo after the transplantation of engineered tissue as a regenerative treatment. In the regenerative medicine of cartilage, it is important to evaluate whether the implanted tissue regenerates as an articular cartilage over time. MRI is the only available non-invasive imaging modality that is utilized for post-operative monitoring and assessment of cartilage regeneration in clinics. Therefore, it is important to evaluate tissue-engineered cartilage using MRI at the preclinical stage as well.
4.7.1 The change in calculated relaxation rate, R2(ECM), using Eq 1 have been found to be positively correlated with tissue growth (3, 6).
SCOPE
1.1 This standard is intended as a standard test method for engineered cartilage tissue growth evaluation using MRI.
1.2 This standard is intended for use in the development of tissue engineering regenerative medical products for cartilage damages, such as in knee, hip, or shoulder joints.
1.3 This standard has been prepared for evaluation of engineered cartilage tissue growth at the preclinical stage and summarizes results from tissue growth evaluation of tissue-engineered cartilage in a few notable cases using water spin-spin relaxation time, T2, in vitro and in vivo in small animal models.
1.4 This standard uses the change in mean T2 values as a function of growth time to evaluate the tissue growth of engineered cartilage.
1.5 This standard provides a method to remove the scaffold contribution to the tissue growth evaluation.
1.6 Information in this standard is intended to be applicable to most porous natural and synthetic polymers used as a scaffold in engineered cartilage, such as alginate, agarose, collagen, chitosan, and poly-lactic-co-glycolic acid (PLGA). However, some materials (both synthetic and natural) may require unique or varied methods of MRI evaluation that are not covered in this test method.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3224 − 17
Standard Test Method for
Evaluating Growth of Engineered Cartilage Tissue using
1
Magnetic Resonance Imaging
This standard is issued under the fixed designation F3224; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This standard is intended as a standard test method for 2.1 The following referenced documents are indispensable
engineered cartilage tissue growth evaluation using MRI. fortheapplicationofthisdocument.Fordatedreferences,only
the edition cited applies. For undated references, the latest
1.2 This standard is intended for use in the development of
edition of the referenced document applies.
tissue engineering regenerative medical products for cartilage
2
damages, such as in knee, hip, or shoulder joints. 2.2 ASTM Standards:
F2312Terminology Relating to Tissue Engineered Medical
1.3 This standard has been prepared for evaluation of
Products
engineered cartilage tissue growth at the preclinical stage and
F2529Guide for in vivo Evaluation of Osteoinductive Po-
summarizes results from tissue growth evaluation of tissue-
tential for Materials Containing Demineralized Bone
engineered cartilage in a few notable cases using water
(DBM)
spin-spin relaxation time, T , in vitro and in vivo in small
2
F2603Guide for Interpreting Images of Polymeric Tissue
animal models.
Scaffolds
1.4 This standard uses the change in mean T values as a
2
F2664Guide for Assessing the Attachment of Cells to
function of growth time to evaluate the tissue growth of
Biomaterial Surfaces by Physical Methods
engineered cartilage.
F2978Guide to Optimize Scan Sequences for Clinical Di-
1.5 This standard provides a method to remove the scaffold agnostic Evaluation of Metal-on-Metal Hip Arthroplasty
Devices using Magnetic Resonance Imaging
contribution to the tissue growth evaluation.
3
2.3 ISO Standard:
1.6 Information in this standard is intended to be applicable
ISO/TR 16379-2014Tissue-engineered medical products —
to most porous natural and synthetic polymers used as a
Evaluation of anisotropic structure of articular cartilage
scaffold in engineered cartilage, such as alginate, agarose,
using DT (Diffusion Tensor)-MR Imaging
collagen, chitosan, and poly-lactic-co-glycolic acid (PLGA).
However, some materials (both synthetic and natural) may
3. Terminology
require unique or varied methods of MRI evaluation that are
3.1 Definitions of Terms Specific to This Standard:
not covered in this test method.
3.1.1 biomaterial, n—any substance (other than a drug),
1.7 This standard does not purport to address all of the
synthetic or natural, that can be used as a system or part of a
safety concerns, if any, associated with its use. It is the
system that treats, augments, or replaces any tissue, organ, or
responsibility of the user of this standard to establish appro-
function of the body. F2664
priate safety, health, and environmental practices and deter-
3.1.2 chondrocyte, n—a cell that has secreted the matrix of
mine the applicability of regulatory limitations prior to use.
cartilage and becomes embedded in it.
1.8 This international standard was developed in accor-
dance with internationally recognized principles on standard-
3.1.3 chondrogenic differentiation, n—the biological pro-
ization established in the Decision on Principles for the cess of stem cells changing their lineage into chondrocytes. If
Development of International Standards, Guides and Recom-
the starting cells are chondrocytes, this term refers to differen-
mendations issued by the World Trade Organization Technical tiation of cells into the same phenotype.
Barriers to Trade (TBT) Committee.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
1
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedical contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee Standards volume information, refer to the standard’s Document Summary page on
F04.44 on Assessment for TEMPs. the ASTM website.
3
CurrenteditionapprovedNov.1,2017.PublishedFebruary2018.DOI:10.1520/ Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
F3224-17. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ---
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.