ASTM D3984-13
(Specification)Standard Specification for Ethane Thermophysical Property Tables
Standard Specification for Ethane Thermophysical Property Tables
ABSTRACT
This specification establishes the formulation of ethane thermophysical property tables for use in the calculation of the pressure-volume-temperature (PVT), thermodynamic, and transport properties of gaseous and liquid ethane for process design and operations. This table will be substantially useful in mathematical models and tables for the thermophysical properties of mixtures containing ethane, such as natural gas.
SCOPE
1.1 The thermophysical property tables for ethane are for use in the calculation of the pressure-volume-temperature (PVT), thermodynamic, and transport properties of ethane for process design and operations. Tables are provided for gaseous and liquid ethane at temperatures between 92 and 600 K at pressures to 20 MPa. Two tables provide properties at the conditions of liquid-vapor equilibrium (saturation properties). A third table provides properties at selected T, p points for the equilibrium phase at those conditions. The tables were developed by the National Institute of Standards and Technology from a Standard Reference Database product REFPROP, version 9.0.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D3984 −13
Standard Specification for
1
Ethane Thermophysical Property Tables
This standard is issued under the fixed designation D3984; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2 These thermophysical property tables are:
3.2.1 ThermophysicalPropertiesofEthaneLiquidatVapor-
1.1 The thermophysical property tables for ethane are for
Liquid Equilibrium, in SI units. See Table 1.
use in the calculation of the pressure-volume-temperature
3.2.2 Thermophysical Properties of Ethane Vapor at Vapor-
(PVT), thermodynamic, and transport properties of ethane for
Liquid Equilibrium, in SI units. See Table 2.
process design and operations. Tables are provided for gaseous
3.2.3 Thermophysical Properties of Ethane Along Isobars,
and liquid ethane at temperatures between 92 and 600 K at
in SI units. See Table 3.
pressures to 20 MPa. Two tables provide properties at the
conditions of liquid-vapor equilibrium (saturation properties). 3.3 The symbols are:
A third table provides properties at selected T, p points for the T, temperature (K)
-1
equilibrium phase at those conditions. The tables were devel- ρ, molar density (mol·l )
-1
oped by the National Institute of Standards and Technology H, molar enthalpy (J·mol )
-1 -1
from a Standard Reference Database product REFPROP, ver- S, molar entropy (J·K ·mol )
-1 -1
C , constant volume molar heat capacity (J·K ·mol )
sion 9.0.
v
-1 -1
C , constant pressure molar heat capacity (J·K ·mol )
p
1.2 The values stated in SI units are to be regarded as
-1
c, speed of sound (m·s )
standard. No other units of measurement are included in this
η, viscosity (µPa·s)
standard.
-1 -1
λ, thermal conductivity (mW·m ·K )
2. Applicability
3.4 The tabulated thermophysical properties are:
-1
ρ, molar density (mol·l )
2.1 These tables apply directly only to pure gaseous ethane.
-1
H, molar enthalpy (J·mol )
However, it is expected that they may find substantial use in
-1 -1
S, molar entropy (J·K ·mol )
mathematical models and tables for the thermophysical prop-
-1 -1
C , constant volume molar heat capacity (J·K ·mol )
erties of mixtures containing ethane. v
-1 -1
C , constant pressure molar heat capacity (J·K ·mol )
p
-1
3. Tables c, speed of sound (m·s )
η, viscosity (µPa·s)
3.1 These tables were produced by equations from a com-
-1 -1
λ, thermal conductivity (mW·m ·K )
puter package, “NIST Standard Reference Database 23; Ref-
erence Fluid Thermodynamic and Transport Properties Data-
4. Additional Information
base (REFPROP): Version 9.0.” A wide selection of units (SI
4.1 Reference state properties are required to calculate
units, engineering units, chemical units) and additional prop-
certain of the thermodynamic properties (enthalpy, entropy,
2
erties are available with this program.
etc.) from an equation of state formulation. The reference state
properties used to generate the tables in this specification are:
enthalpy, H, and entropy, S, at the Normal Boiling Point;
1
This specification is under the jurisdiction of ASTM Committee D03 on
184.57K and 0.10133MPa (H = 14716 J/mol and S = 79.731
Gaseous Fuels and is the direct responsibility of Subcommittee D03.08 on
Thermophysical Properties.
J/(mol K)). The molar mass of ethane is 30.069 g/mol.
Current edition approved May 1, 2013. Published May 2013. Originally
approved in 1982. Last previous edition approved in 2008 as D3984 – 08. DOI: 5. Keywords
10.1520/D3984-13.
2 5.1 ethane gas tables; natural gas; thermodynamic proper-
Available from Standard Reference Data, National Institute of Standards and
Technology (NIST), 100 Bureau Drive, Stop 3460, Gaithersburg, MD 20899. ties of ethane; transport properties of ethane
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D3984−13
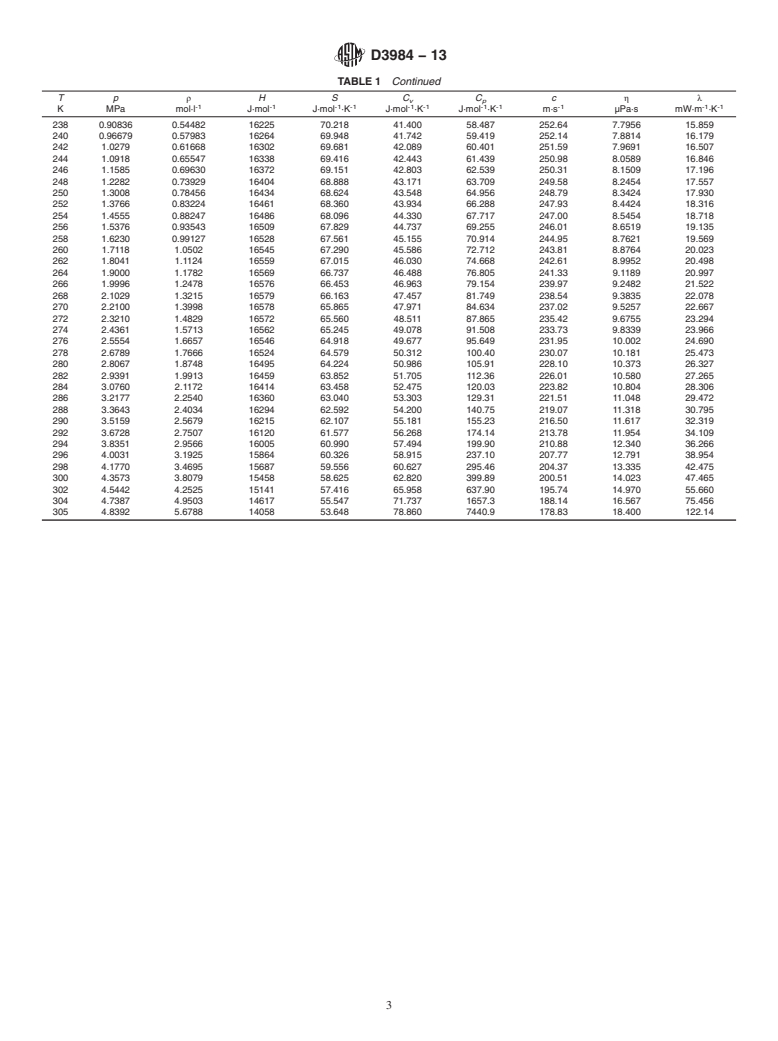
TABLE 1 Thermophysical Properties of Ethane Liquid at Vapor-Liquid Equilibrium
Tp ρ HS C C c ηλ
v p
-1 -1 -1 -1 -1 -1 -1 -1 -1 -1 -1
K MPa mol·l J·mol J·mol ·K J·mol ·K J·mol ·K m·s µPa·s mW·m ·K
90.4 1.1518E-06 1.5324E-06 11295 148.09 26.811 35.126 180.97 3.0436 2.9100
92 1.7410E-06 2.2760E-06 11351 145.27 26.905 35.220 182.48 3.0888 3.0000
94 2.8559E-06 3.6541E-06 11421 141.92 27.024 35.339 184.36 3.1453 3.1131
96 4.5809E-06 5.7391E-06 11492 138.73 27.143 35.458 186.22 3.2020 3.2268
98 7.1951E-06 8.8304E-06 11563 135.71 27.263 35.578 188.05 3.2588 3.3412
100 1.1081E-05 1.3327E-05 11634 132.84 27.384 35.699 189.86 3.3157 3.4563
102 1.6752E-05 1.9754E-05 11706 130.11 27.505 35.821 191.65 3.3729 3.5723
104 2.4891E-05 2.8787E-
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D3984 − 08 D3984 − 13
Standard Specification for
1
Ethane Thermophysical Property Tables
This standard is issued under the fixed designation D3984; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 The thermophysical property tables for ethane are for use in the calculation of the pressure-volume-temperature (PVT),
thermodynamic, and transport properties of ethane for process design and operations. Tables are provided for gaseous and liquid
ethane at temperatures between 92 and 600 K at pressures to 20 MPa. One table providesTwo tables provide properties at the
conditions of liquid-vapor equilibrium (saturation properties). The otherA third table provides properties at selected T,p points for
the equilibrium phase at those conditions. The tables were developed by the National Institute of Standards and Technology from
a Standard Reference Database product REFPROP, version 8.0.9.0.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
2. Applicability
2.1 These tables apply directly only to pure gaseous ethane. However, it is expected that they may find substantial use in
mathematical models and tables for the thermophysical properties of mixtures containing ethane.
1
This specification is under the jurisdiction of ASTM Committee D03 on Gaseous Fuels and is the direct responsibility of Subcommittee D03.08 on Thermophysical
Properties.
Current edition approved Dec. 1, 2008May 1, 2013. Published January 2009May 2013. Originally approved in 1982. Last previous edition approved in 20032008 as
D3984 – 93 (2003).D3984 – 08. DOI: 10.1520/D3984-08.10.1520/D3984-13.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D3984 − 13
3. Tables
3.1 These tables were produced by equations from a computer package, “NIST Standard Reference Database 23; Reference
Fluid Thermodynamic and Transport Properties Database (REFPROP): Version 9.0.” A wide selection of units (SI units,
2
engineering units, chemical units) and additional properties are available with this program.
3.2 These thermophysical property tables are:
3.2.1 Thermophysical Properties of Coexisting Gaseous and Liquid Ethane, Ethane Liquid at Vapor-Liquid Equilibrium, in SI
units. See Table 1.
3.2.2 Thermophysical Properties of Ethane Along Isobars, Vapor at Vapor-Liquid Equilibrium, in SI units. See Table 2.
3.2.3 Thermophysical Properties of Ethane Along Isobars, in SI units. See Table 3.
3.3 The symbols are:
T, temperature (K)
-1
ρ, molar density (mol·l )
-1
H, molar enthalpy (J·mol )
-1 -1
S, molar entropy (J·K ·mol )
-1 -1
C , constant volume molar heat capacity (J·K ·mol )
v
-1 -1
C , constant pressure molar heat capacity (J·K ·mol )
p
-1
c, speed of sound (m·s )
η, viscosity (μPa·s)
-1 -1
λ, thermal conductivity (mW·m ·K )
3.4 The tabulated thermophysical properties are:
-1
ρ, molar density (mol·l )
-1
H, molar enthalpy (J·mol )
-1 -1
S, molar entropy (J·K ·mol )
-1 -1
C , constant volume molar heat capacity (J·K ·mol )
v
-1 -1
C , constant pressure molar heat capacity (J·K ·mol )
p
-1
c, speed of sound (m·s )
η, viscosity (μPa·s)
-1 -1
λ, thermal conductivity (mW·m ·K )
3.3 These tables were produced by equations from a computer package, “NIST Standard Reference Database 23; Reference
Fluid Thermodynamic and Transport Properties Database (REFPROP): Version =8.0” A wide selection of units (SI units,
2
engineering units, chemical units) is available with this program.
4. Additional Information
4.1 Reference state properties are required to calculate certain of the thermodynamic properties (enthalpy, entropy, etc.) from
an equation of state formulation. The reference state properties used to generate the tables in this specification are: enthalpy, H,
and entropy, S, at 298.15 K and 0.101325 MPa the Normal Boiling Point; 184.57K and 0.10133MPa (H = 11874.2 = 14716 J/mol
and S = 221.116 J/(mol K). = 79.731 J/(mol K)). The molar mass of ethane is 30.069 g/mol.
5. Keywords
5.1 ethane gas tables; natural gas; thermodynamic properties of ethane; transport properties of ethane
2
Available from Standard Reference Data, National Institute of Standards and Technology (NIST),
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.