ASTM D4468-85(2011)
(Test Method)Standard Test Method for Total Sulfur in Gaseous Fuels by Hydrogenolysis and
Rateometric Colorimetry
Standard Test Method for Total Sulfur in Gaseous Fuels by Hydrogenolysis and<br> Rateometric Colorimetry
SIGNIFICANCE AND USE
This test method can be used to determine specification, or regulatory compliance to requirements, for total sulfur in gaseous fuels. In gas processing plants, sulfur can be a contaminant and must be removed before gas is introduced into gas pipelines. In petrochemical plants, sulfur is a poison for many catalysts and must be reduced to acceptable levels, usually in the range from 0.01 to 1 ppm/v. This test method may also be used as a quality-control tool for sulfur determination in finished products, such as propane, butane, ethane, and ethylene.
SCOPE
1.1 This test method covers the determination of sulfur gaseous fuels in the range from 0.001 to 20 parts per million by volume (ppm/v).
1.2 This test method may be extended to higher concentration by dilution.
This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in 6.7, 6.8, and 7.3.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D4468 − 85(Reapproved 2011)
Standard Test Method for
Total Sulfur in Gaseous Fuels by Hydrogenolysis and
Rateometric Colorimetry
This standard is issued under the fixed designation D4468; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope metric reaction of H S with lead acetate. Units used are ppm/v,
which is equivalent to micromoles/mole.
1.1 This test method covers the determination of sulfur
gaseousfuelsintherangefrom0.001to20partspermillionby
4. Significance and Use
volume (ppm/v).
4.1 This test method can be used to determine specification,
1.2 This test method may be extended to higher concentra-
or regulatory compliance to requirements, for total sulfur in
tion by dilution.
gaseous fuels. In gas processing plants, sulfur can be a
1.3 The values stated in SI units are to be regarded as
contaminantandmustberemovedbeforegasisintroducedinto
standard. No other units of measurement are included in this
gas pipelines. In petrochemical plants, sulfur is a poison for
standard.
many catalysts and must be reduced to acceptable levels,
1.4 This standard may involve hazardous materials,
usually in the range from 0.01 to 1 ppm/v. This test method
operations, and equipment. This standard does not purport to
may also be used as a quality-control tool for sulfur determi-
address all of the safety concerns associated with its use. It is
nation in finished products, such as propane, butane, ethane,
the responsibility of the user of this standard to establish
and ethylene.
appropriate safety and health practices and determine the
applicability of regulatory limitations prior to use. Specific
5. Apparatus
precautionary statements are given in 6.7, 6.8, and 7.3.
5.1 Pyrolysis Furnace—A furnace that can provide an
adjustable temperature of 900 to 1300°C in a quartz or ceramic
2. Referenced Documents
tube of 5 mm or larger tube (ID) is required for pyrolysis of the
2.1 ASTM Standards:
sample. (See Fig. 1.) The flow system is to be a fluorocarbon
D1193 Specification for Reagent Water
orothermaterialinerttoH Sandothersulfurcompounds.(See
D1914 PracticeforConversionUnitsandFactorsRelatingto
Fig. 1.)
Sampling and Analysis of Atmospheres
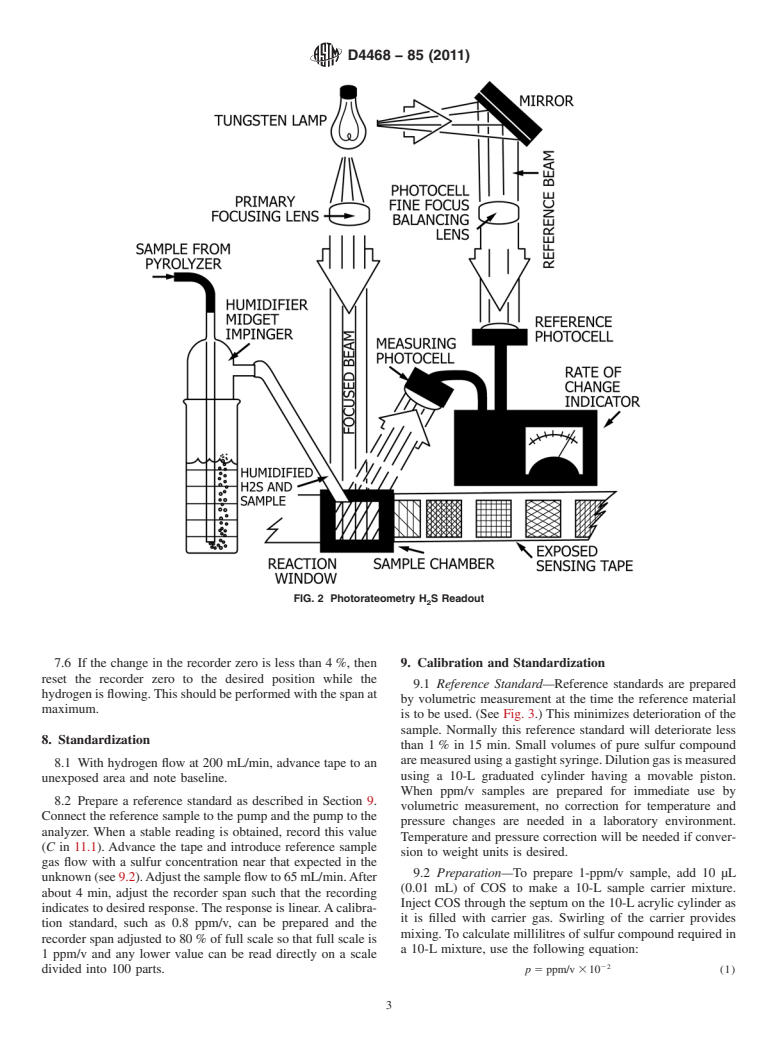
5.2 Rateometric H S Readout—Hydrogenolysis products
D4045 Test Method for Sulfur in Petroleum Products by
contain H S in proportion to sulfur in the sample. The H S
2 2
Hydrogenolysis and Rateometric Colorimetry
concentration is determined by measuring rate of change of
reflectance of a tape impregnated with lead acetate caused by
3. Summary of Test Method
darkening when lead sulfide is formed. Rateometric
3.1 The sample is introduced at a constant rate into a
electronics, adapted to provide first derivative output, allows
flowing hydrogen stream in a hydrogenolysis apparatus. The
sufficient sensitivity to measure to 0.001 ppm/v. (See Fig. 2.)
sampleandhydrogenarepyrolyzedatatemperatureof1000°C
5.3 Recorder—A suitable chart recorder may be used for a
or above, to convert sulfur compounds to hydrogen sulfide
permanent record of analysis.
(H S). Readout is by the rateometric detection of the colori-
6. Reagents and Materials
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
6.1 Purity of Chemicals—Reagent grade unless specified
Fuels and is the direct responsibility of Subcommittee D03.05 on Determination of
otherwise.
Special Constituents of Gaseous Fuels.
Current edition approved Nov. 1, 2011. Published December 2011. Originally
6.2 Purity of Water—Unless otherwise indicated, reference
approved in 1985. Last previous edition approved in 2006 as D4468–85 (2006).
to water shall be understood to mean Type II, reagent grade
DOI: 10.1520/D4468-85R11.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or water, conforming to Specification D1193.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
6.3 Sensing Tape—Lead acetate impregnated analytical
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. quality filter paper shall be used.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D4468 − 85 (2011)
FIG. 1 Hydrogenolysis Flow Diagram
6.4 Acetic Acid (5 %)—Mix 1 part by volume reagent grade 7. Preparation of Apparatus
glacial acetic acid with 19 parts water to prepare 5 % acetic
7.1 Turnonthefurnaceandallowtemperaturetostabilizeat
acid solution.
1000°C. If thiophenic sulfur could be present, use 1300°C
6.5 Gastight Syringe—A gastight 0.1- and 0.5-mL syringe
temperature setting.
for preparing calibration standard. Volumetric measurement
NOTE 1—Reduced operating temperature extends furnace life. Thio-
accuracy of the syringe shall be 1 % or better.
phenic compound conversion increases from about 60 % at 1000°C to
6.6 Piston Cylinder—Usea10-Lacryliccylinderwithafree
100 % at 1300°C.
moving piston and silicone rubber “O” ring lubricated with a
7.2 Connect all flow tubing between components and fill
free-flowing silicone lubricant. This cylinder is used to prepare
humidifier inside the cabinet to 30 mL with a 5 % by volume
ppm/v calibration samples volumetrically.
acetic acid solution. Purge all flow systems with inert gas then
6.7 Carbonyl Sulfide (COS)—Alecture bottle of COS, 99 %
close valve. Check all connections for leaks with soap solution
purity, with a needle valve connected to the lecture bottle
and repair any leaks. Connect hydrogen and set flow at 200
outlet. Connect 2 ft of tygon tubing to allow insertion of a
mL/min and allow temperature to stabilize. Sample flow must
hypodermic syringe to withdraw pure COS while tubing is
be ⁄3 or less of the H flow. Total flow can be up to 500
purged from the lecture bottle. Other sulfur compounds can be
mL/min, except when the sample has thiophenic compounds
used with adequate odor control. If the sulfur compound has
that require 200 mL/min of H flow for conversion. Make final
two sulfur atoms per molecule, reduce the volume by one half.
temperature adjustment to 1000 6 15°C or a minimum 1300°C
(Warning —Work with COS should be done in a well-
if the sample contains thiophenic sulfur compounds.
ventilated area, or under a fume hood.)
7.3 Install sensing tape and turn H S readout analyzer on.
6.8 Hydrogen Gas—Use sulfur-free hydrogen of laboratory
Use adequate safety precautions in handling lead acetate tape.
grade. (Warning—Hydrogen has wide explosive limits when
mixed with air. See 1.4 regarding precautions.)
7.4 Adjust the zero of the analyzer indicator meter (and
recorder if used) to desired position with no flow. This should
6.9 Carrier Gas for Calibration Standards—Use sulfur-free
be performed with span at maximum.
laboratory grade bottled gas of the same type or similar density
as the gas to be analyzed or calibrate the flowmeter to establish
7.5 Test hydrogen purity by turning on hydrogen flow and
correct flow setting for an available carrier gas. Test, as in 7.5,
noting any change in zero position after 5 min. If the reading
adding the carrier gas flow to the hydrogen flow.
is upscale from the zero set point by greater than 4 %, then the
6.10 Purge Gas—Sulfur-free purge gas, nitrogen, CO,or hydrogen source should be suspect as not being sulfur free and
other inert gas. Commercial grade cylinder gas is satisfactory. should be changed.
----------------------
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.