ASTM E1563-21a
(Guide)Standard Guide for Conducting Short-Term Chronic Toxicity Tests with Echinoid Embryos
Standard Guide for Conducting Short-Term Chronic Toxicity Tests with Echinoid Embryos
SIGNIFICANCE AND USE
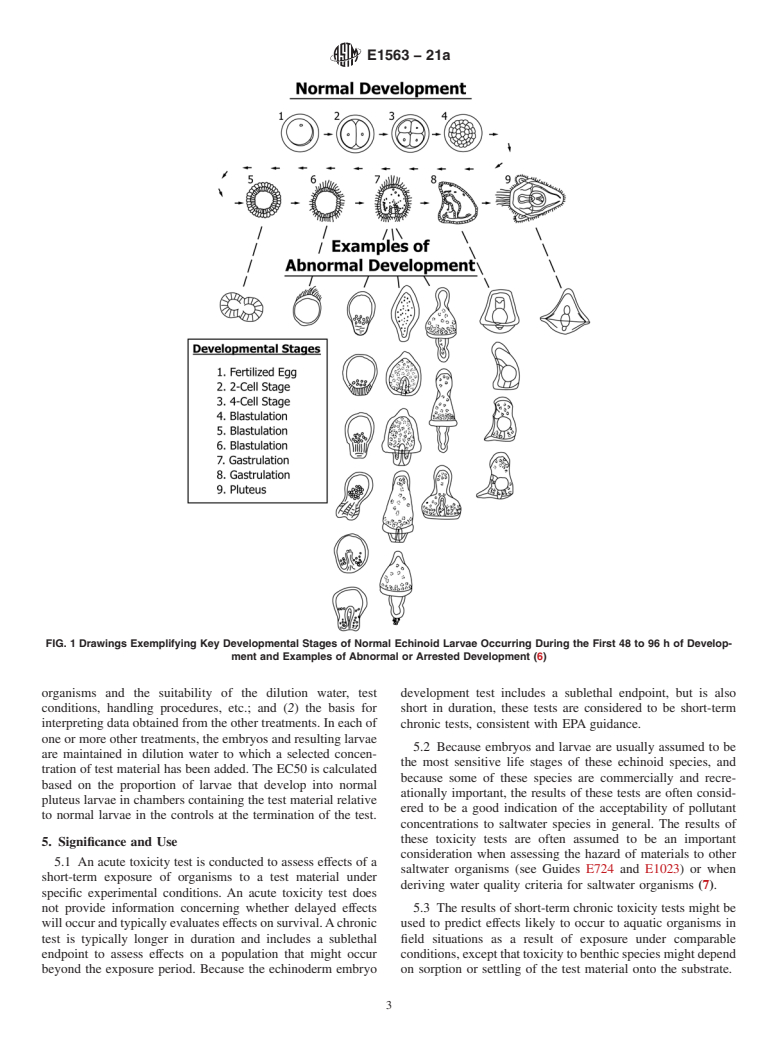
5.1 An acute toxicity test is conducted to assess effects of a short-term exposure of organisms to a test material under specific experimental conditions. An acute toxicity test does not provide information concerning whether delayed effects will occur and typically evaluates effects on survival. A chronic test is typically longer in duration and includes a sublethal endpoint to assess effects on a population that might occur beyond the exposure period. Because the echinoderm embryo development test includes a sublethal endpoint, but is also short in duration, these tests are considered to be short-term chronic tests, consistent with EPA guidance.
5.2 Because embryos and larvae are usually assumed to be the most sensitive life stages of these echinoid species, and because some of these species are commercially and recreationally important, the results of these tests are often considered to be a good indication of the acceptability of pollutant concentrations to saltwater species in general. The results of these toxicity tests are often assumed to be an important consideration when assessing the hazard of materials to other saltwater organisms (see Guides E724 and E1023) or when deriving water quality criteria for saltwater organisms (7).
5.3 The results of short-term chronic toxicity tests might be used to predict effects likely to occur to aquatic organisms in field situations as a result of exposure under comparable conditions, except that toxicity to benthic species might depend on sorption or settling of the test material onto the substrate.
5.4 The results of short-term chronic tests might be used to compare the sensitivities of different species and the acute toxicities of different test materials, and to determine the effects of various environmental factors on the results of such tests.
5.5 The results of short-term chronic toxicity tests might be useful for studying the biological availability of, and structure-activity relationships between, t...
SCOPE
1.1 This guide covers procedures for obtaining laboratory data concerning the short-term chronic effects of a test material on echinoderm embryos and the resulting larvae (sea urchins and sand dollars) during static 48- to 96-h exposures. These procedures have generally been used with U.S. East Coast (Arbacia punctulata and Strongylocentrotus droebachiensis ) (1)3 and West Coast species (Strongylocentrotus purpuratus, S. droebachiensis, and Dendraster excentricus) (2). The basic procedures described in this guide first originated in Japan and Scandanavia (3), and parallel procedures have been used with foreign species, especially in Japan and the Mediterranean (4). These procedures will probably be useful for conducting static toxicity tests with embryos of other echinoid species, although modifications might be necessary.
1.2 Other modifications of these procedures might be justified by special needs or circumstances. Although using procedures appropriate to a particular species or special needs and circumstances is more important than following prescribed procedures, the results of tests conducted by using unusual procedures are not likely to be comparable with those of many other tests. The comparison of results obtained by using modified and unmodified versions of these procedures might provide useful information concerning new concepts and procedures for conducting tests starting with embryos of echinoids.
1.3 These procedures are applicable to most chemicals, either individually or in formulations, commercial products, or known mixtures. With appropriate modifications, these procedures can be used to conduct tests on temperature, dissolved oxygen, and pH and on such materials as aqueous effluents (see also Guide E1192), leachates, oils, particulate matter, surface waters, effluents, and sediments (Annex A1). Renewal tests might be preferable to static tests for materials that have a high oxygen demand, are...
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E1563 − 21a
Standard Guide for
Conducting Short-Term Chronic Toxicity Tests with Echinoid
1,2
Embryos
This standard is issued under the fixed designation E1563; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* also Guide E1192), leachates, oils, particulate matter, surface
waters, effluents, and sediments (Annex A1). Renewal tests
1.1 This guide covers procedures for obtaining laboratory
mightbepreferabletostatictestsformaterialsthathaveahigh
dataconcerningtheshort-termchroniceffectsofatestmaterial
oxygen demand, are highly volatile, are rapidly transformed
on echinoderm embryos and the resulting larvae (sea urchins
biologically or chemically in aqueous solution, or are removed
and sand dollars) during static 48- to 96-h exposures. These
from test solutions in substantial quantities by the test cham-
procedures have generally been used with U.S. East Coast
bers or organisms during the test.
(Arbacia punctulata and Strongylocentrotus droebachiensis )
3
(1) andWestCoastspecies(Strongylocentrotus purpuratus, S. 1.4 Results of short-term chronic toxicity tests with echi-
noid embryos should usually be reported as the 50% effect
droebachiensis, and Dendraster excentricus) (2). The basic
procedures described in this guide first originated in Japan and concentration (EC50) based on the total abnormally developed
embryos and larvae. In some situations, it might only be
Scandanavia (3), and parallel procedures have been used with
foreign species, especially in Japan and the Mediterranean (4). necessary to determine whether a specific concentration is
toxic to embryos or whether the EC50 is above or below a
These procedures will probably be useful for conducting static
toxicity tests with embryos of other echinoid species, although specific concentration.
modifications might be necessary.
1.5 This guide is arranged as follows:
1.2 Other modifications of these procedures might be justi-
fied by special needs or circumstances.Although using proce-
dures appropriate to a particular species or special needs and
Section
Scope 1
circumstances is more important than following prescribed
Referenced Documents 2
procedures, the results of tests conducted by using unusual
Terminology 3
procedures are not likely to be comparable with those of many
Summary of Guide 4
Significance and Use 5
other tests. The comparison of results obtained by using
Apparatus 6
modified and unmodified versions of these procedures might
Facilities 6.1
provide useful information concerning new concepts and
Construction Materials 6.2
Test Chambers 6.3
procedures for conducting tests starting with embryos of
Cleaning 6.4
echinoids.
Acceptability 6.5
Safety Precautions 7
1.3 These procedures are applicable to most chemicals,
Dilution Water 8
either individually or in formulations, commercial products, or
Requirements 8.1
known mixtures. With appropriate modifications, these proce- Source 8.2
Treatment 8.3
dures can be used to conduct tests on temperature, dissolved
Characterization 8.4
oxygen,andpHandonsuchmaterialsasaqueouseffluents(see
Test Material 9
General 9.1
Stock Solution 9.2
Test Concentration(s) 9.3
1
A Standard Guide is a document, developed using the consensus mechanisms
Test Organisms 10
of ASTM that provides guidance for the selection of procedures to accomplish a
Species 10.1
specific test, but which does not stipulate specific procedures.
Age 10.2
2
ThisguideisunderthejurisdictionofASTMCommitteeE50onEnvironmental Source of Embryos 10.3
Assessment, Risk Management and CorrectiveAction and is the direct responsibil- Handling 10.4
Test Animal Source and Condition 10.5
ity of Subcommittee E50.47 on Biological Effects and Environmental Fate.
Spawning and Fertilization 10.6
Current edition approved Nov. 1, 2021. Published January 2022. Originally
Quality 10.7
approved in 1995. Last previous edition approved in 2021 as E1563–21. DOI:
Procedure 11
10.1520/E1563-21A.
3
Experimental Design 11.1
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
Dissolved Oxygen 11.2
this standard.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E1563 − 21a
E1525GuideforDesigningBiologicalTestswithSediments
Temperature 11.3
Beginning the Test 11.4
E1706TestMethodforMeasuringtheToxicityofSed
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E1563 − 21 E1563 − 21a
Standard Guide for
Conducting Short-Term Chronic Toxicity Tests with Echinoid
1,2
Embryos
This standard is issued under the fixed designation E1563; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope Scope*
1.1 This guide covers procedures for obtaining laboratory data concerning the short-term chronic effects of a test material on
echinoderm embryos and the resulting larvae (sea urchins and sand dollars) during static 48- to 96-h exposures. These procedures
3
have generally been used with U.S. East Coast (Arbacia punctulata and Strongylocentrotus droebachiensis ) (1) and West Coast
species (Strongylocentrotus purpuratus, S. droebachiensis, and Dendraster excentricus) (2). The basic procedures described in this
guide first originated in Japan and Scandanavia (3), and parallel procedures have been used with foreign species, especially in
Japan and the Mediterranean (4). These procedures will probably be useful for conducting static toxicity tests with embryos of
other echinoid species, although modifications might be necessary.
1.2 Other modifications of these procedures might be justified by special needs or circumstances. Although using procedures
appropriate to a particular species or special needs and circumstances is more important than following prescribed procedures, the
results of tests conducted by using unusual procedures are not likely to be comparable with those of many other tests. The
comparison of results obtained by using modified and unmodified versions of these procedures might provide useful information
concerning new concepts and procedures for conducting tests starting with embryos of echinoids.
1.3 These procedures are applicable to most chemicals, either individually or in formulations, commercial products, or known
mixtures. With appropriate modifications, these procedures can be used to conduct tests on temperature, dissolved oxygen, and pH
and on such materials as aqueous effluents (see also Guide E1192), leachates, oils, particulate matter, surface waters, effluents, and
sediments (Annex A1). Renewal tests might be preferable to static tests for materials that have a high oxygen demand, are highly
volatile, are rapidly transformed biologically or chemically in aqueous solution, or are removed from test solutions in substantial
quantities by the test chambers or organisms during the test.
1.4 Results of short-term chronic toxicity tests with echinoid embryos should usually be reported as the 50 % effect concentration
(EC50) based on the total abnormally developed embryos and larvae. In some situations, it might only be necessary to determine
whether a specific concentration is toxic to embryos or whether the EC50 is above or below a specific concentration.
1.5 This guide is arranged as follows:
1
A Standard Guide is a document, developed using the consensus mechanisms of ASTM that provides guidance for the selection of procedures to accomplish a specific
test, but which does not stipulate specific procedures.
2
This guide is under the jurisdiction of ASTM Committee E50 on Environmental Assessment, Risk Management and Corrective Action and is the direct responsibility
of Subcommittee E50.47 on Biological Effects and Environmental Fate.
Current edition approved Jan. 15, 2021Nov. 1, 2021. Published February 2021January 2022. Originally approved in 1995. Last previous edition approved in 20122021
as E1563 – 98 (2012).E1563 – 21. DOI: 10.1520/E1563-21.10.1520/E1563-21A.
3
The boldface numbers in parentheses refer to the list of references at the end of this standard.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E1563 − 21a
Section
Scope 1
Referenced Documents 2
Terminology 3
Summary of Guide 4
Significance and Use 5
Apparatus 6
Facilities 6.1
Construction Materials 6.2
Test Chambers 6.3
Cleaning 6.4
Acceptability 6.5
Safety Precautions 7
Dilution Water 8
Requirements 8.1
Source 8.2
Treatment 8.3
Characterization 8.4
Test Material 9
General 9.1
Stock Solution 9.2
Test Concentration(s) 9.3
Test Organisms 10
Species 10.1
Age 10.2
Source of Embryos 10.3
Handling 10.4
Test Animal Source
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.