ASTM D7318-11
(Test Method)Standard Test Method for Existent Inorganic Sulfate in Ethanol by Potentiometric Titration
Standard Test Method for Existent Inorganic Sulfate in Ethanol by Potentiometric Titration
SIGNIFICANCE AND USE
Ethanol is used as a blending agent added to gasoline. Sulfates are indicated in filter plugging deposits and fuel injector deposits. When fuel ethanol is burned, sulfates may contribute to sulfuric acid emissions. Ethanol acceptability for use depends on the sulfate content. Sulfate content, as measured by this test method, can be used as one measure of determination of the acceptability of ethanol for automotive spark-ignition engine fuel use.
SCOPE
1.1 This test method covers a potentiometric titration procedure for determining the existent inorganic sulfate content of hydrous, anhydrous ethanol, and anhydrous denatured ethanol, which is added as a blending agent with spark ignition fuels. It is intended for the analysis of denatured ethanol samples containing between 1.0–20 mg/kg existent inorganic sulfate.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Material Safety Data Sheets are available for reagents and materials. Review them for hazards prior to usage.
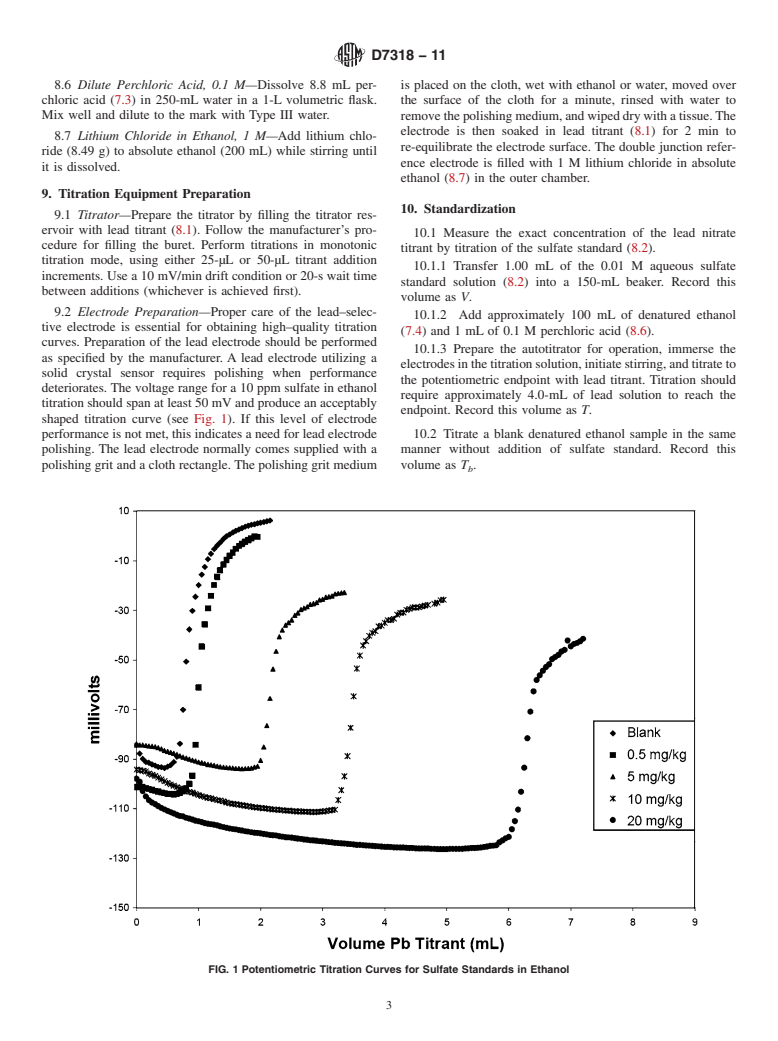
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D7318 − 11
StandardTest Method for
Existent Inorganic Sulfate in Ethanol by Potentiometric
1
Titration
This standard is issued under the fixed designation D7318; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* 3.1.1 existent inorganic sulfate, n—sulfate species present
as sulfuric acid, ionic salts of this acid, or mixtures of these.
1.1 This test method covers a potentiometric titration pro-
3.1.1.1 Discussion—Specifically in this test method, inor-
cedure for determining the existent inorganic sulfate content of
ganic sulfate is present as sulfate in ethanol.
hydrous, anhydrous ethanol, and anhydrous denatured ethanol,
which is added as a blending agent with spark ignition fuels. It
4. Summary of Test Method
is intended for the analysis of denatured ethanol samples
4.1 An ethanol sample containing inorganic sulfate is
containing between 1.0–20 mg/kg existent inorganic sulfate.
titrated in ethanolic medium with a standard lead nitrate
1.2 The values stated in SI units are to be regarded as
solution. Lead sulfate precipitate is formed during the titration.
standard. No other units of measurement are included in this
Perchloric acid is added to remove possible interference from
standard.
carbonate. The endpoint is signaled by an increase in lead ion
1.3 This standard does not purport to address all of the
activity, as measured by a lead–selective electrode.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
5. Significance and Use
priate safety and health practices and determine the applica-
5.1 Ethanol is used as a blending agent added to gasoline.
bility of regulatory limitations prior to use. Material Safety
Sulfates are indicated in filter plugging deposits and fuel
Data Sheets are available for reagents and materials. Review
injector deposits. When fuel ethanol is burned, sulfates may
them for hazards prior to usage.
contribute to sulfuric acid emissions. Ethanol acceptability for
use depends on the sulfate content. Sulfate content, as mea-
2. Referenced Documents
sured by this test method, can be used as one measure of
2
2.1 ASTM Standards:
determination of the acceptability of ethanol for automotive
D1193 Specification for Reagent Water
spark-ignition engine fuel use.
D4052 Test Method for Density, Relative Density, and API
Gravity of Liquids by Digital Density Meter
6. Apparatus
D4057 Practice for Manual Sampling of Petroleum and
6.1 Potentiometric Titration Assembly—A titration assem-
Petroleum Products
bly consisting of an automatic titrator fitted with a lead
D4177 Practice for Automatic Sampling of Petroleum and
ion-selective electrode, a double-junction reference electrode,
Petroleum Products
buret, and stirring is used. Stirring may be accomplished by
D6299 Practice for Applying Statistical Quality Assurance
means of magnetic or propeller type stirrer mechanisms. The
and Control Charting Techniques to Evaluate Analytical
buret size should ideally be 10 or 20 mL.
Measurement System Performance
6.2 Reference Electrode—A double junction reference elec-
3. Terminology
trode with the inner electrode composed of silver/silver chlo-
3.1 Definitions:
ride with a potassium chloride solution as internal electrolyte.
The external solution is composed of 1 M lithium chloride in
1
This test method is under the jurisdiction of ASTM Committee D02 on
ethanol. This configuration is used to prevent silver ion, a lead
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
electrodepoison,fromleachingintotheanalytesolutionduring
D02.03 on Elemental Analysis.
titration. Preferred electrolytes for use in double junction
Current edition approved July 1, 2011. Published August 2011. Originally
electrodes may vary with the manufacturer; use the manufac-
approved in 2007. Last previous edition approved in 2007 as D7318–07. DOI:
10.1520/D7318-07.
turer’s recommended electrolytes for the application. Other
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
types of reference electrodes may be considered with some
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
caveats (for example, single junction, combination, or glassy
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. carbon), but the data presented in this test method were
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United State
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D7318–07 Designation:D7318–11
Standard Test Method for
TotalExistent Inorganic Sulfate in Ethanol by Potentiometric
1
Titration
This standard is issued under the fixed designation D7318; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope*
1.1 This test method covers a potentiometric titration procedure for determining the totalexistent inorganic sulfate content of
hydrous, anhydrous ethanol, and anhydrous denatured ethanol, which is added as a blending agent with spark ignition fuels. It is
intended for the analysis of denatured ethanol samples containing between 1.0–20 mg/kg totalexistent inorganic sulfate.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parenthesesNo other units of
measurement are for information only. included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.Material Safety Data Sheets are available for reagents and materials. Review them for hazards prior to
usage.
2. Referenced Documents
2
2.1 ASTM Standards:
D1193 Specification for Reagent Water
D4052 Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter
D4057 Practice for Manual Sampling of Petroleum and Petroleum Products
D4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
D6299 Practice for Applying Statistical Quality Assurance and Control Charting Techniques to Evaluate Analytical
Measurement System Performance
3. Terminology
3.1 Definitions:
3.1.1 inorganic sulfateexistent inorganic sulfate, n—sulfate species present as sulfuric acid, ionic salts of this acid, or mixtures
of these.
3.1.1.1 Discussion—Specifically in this test method, inorganic sulfate is present as sulfate in ethanol.
4. Summary of Test Method
4.1 An ethanol sample containing inorganic sulfate is titrated in ethanolic medium with a standard lead nitrate solution. Lead
sulfate precipitate is formed during the titration. Perchloric acid is added to remove possible interference from carbonate. The
endpoint is signaled by an increase in lead ion activity, as measured by a lead–selective electrode.
5. Significance and Use
5.1 Ethanol is used as a blending agent added to gasoline. Sulfates are indicated in filter plugging deposits and fuel injector
deposits. When fuel ethanol is burned, sulfates may contribute to sulfuric acid emissions. Ethanol acceptability for use depends
on the sulfate content. Sulfate content, as measured by this test method, can be used as one measure of determination of the
acceptability of ethanol for automotive spark-ignition engine fuel use.
1
This test method is under the jurisdiction of ASTM Committee D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.03 on
Elemental Analysis.
Current edition approved Feb. 1, 2007. Published March 2007. DOI: 10.1520/D7318-07.
Current edition approved July 1, 2011. Published August 2011. Originally approved in 2007. Last previous edition approved in 2007 as D7318–07. DOI:
10.1520/D7318-07.
2
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
D7318–11
6. Apparatus
6.1 Potentiometric Titration Assembly—A titration assembly consisting of an automatic titrator fitted with a lead ion-selective
electrode, a double-junction reference electrode, buret, and stirring is used. Stirring may be accomplished by means of magnetic
or propeller type stirrer mechanisms. The buret size should ideally be 10 or 20 mL.
6.2 Reference Electrode—Adoublejunctionreferenceelectrodewiththeinnerelectrodecomposedofsilver/silverchloridewith
a potassium chloride solution as internal electrolyte. The external solution is compose
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.