ASTM E1719-12(2018)
(Test Method)Standard Test Method for Vapor Pressure of Liquids by Ebulliometry (Withdrawn 2023)
Standard Test Method for Vapor Pressure of Liquids by Ebulliometry (Withdrawn 2023)
SIGNIFICANCE AND USE
5.1 Vapor pressure is a fundamental thermodynamic property of a liquid. Vapor pressure and boiling temperature data are required for material safety data sheets (MSDS), the estimation of volatile organic compounds (VOC), and other needs related to product safety. Vapor pressures are important for prediction of the transport of a chemical in the environment; see Test Method E1194.
SCOPE
1.1 This test method describes procedures for determination of the vapor pressure of liquids by ebulliometry (boiling point measurements). It is applicable to pure liquids and azeotropes that have an atmospheric boiling point between 285 and 575 K and that can be condensed completely and returned to the ebulliometer boiler, that is, all materials must be condensable at total reflux. Liquid mixtures may be studied if they do not contain non-condensable components. Liquid mixtures that contain trace amounts of volatile but completely condensable components may also be studied, but they will produce vapor pressure data of greater uncertainty. Boiling point temperatures are measured at applied pressures of 1.0 to 100 kPa (7.5 to 760 torr).
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 There is no ISO equivalent to this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Section 8.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E1719 − 12 (Reapproved 2018)
Standard Test Method for
Vapor Pressure of Liquids by Ebulliometry
This standard is issued under the fixed designation E1719; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D2879 Test Method for Vapor Pressure-Temperature Rela-
tionship and Initial Decomposition Temperature of Liq-
1.1 This test method describes procedures for determination
uids by Isoteniscope
of the vapor pressure of liquids by ebulliometry (boiling point
E1 Specification for ASTM Liquid-in-Glass Thermometers
measurements). It is applicable to pure liquids and azeotropes
E177 Practice for Use of the Terms Precision and Bias in
that have an atmospheric boiling point between 285 and 575 K
ASTM Test Methods
and that can be condensed completely and returned to the
E1142 Terminology Relating to Thermophysical Properties
ebulliometer boiler, that is, all materials must be condensable
E1194 Test Method for Vapor Pressure
at total reflux. Liquid mixtures may be studied if they do not
E1970 Practice for Statistical Treatment of Thermoanalytical
contain non-condensable components. Liquid mixtures that
Data
contain trace amounts of volatile but completely condensable
components may also be studied, but they will produce vapor
3. Terminology
pressure data of greater uncertainty. Boiling point temperatures
are measured at applied pressures of 1.0 to 100 kPa (7.5 to 760
3.1 Definitions:
torr). 3.1.1 The following terms are applicable to this test method
and can be found in Terminology E1142; boiling temperature
1.2 The values stated in SI units are to be regarded as
and vapor pressure.
standard. No other units of measurement are included in this
3.1.2 For definitions of other terms used in this test method,
standard.
refer to Terminology E1142.
1.3 There is no ISO equivalent to this standard.
3.2 Definitions of Terms Specific to This Standard:
1.4 This standard does not purport to address all of the
3.2.1 ebulliometer—a one-stage, total-reflux boiler designed
safety concerns, if any, associated with its use. It is the
to minimize superheating of the boiling liquid.
responsibility of the user of this standard to establish appro-
3.2.2 manostat—a device for maintaining constant vacuum
priate safety, health, and environmental practices and deter-
or pressure.
mine the applicability of regulatory limitations prior to use.
3.2.3 superheating—the act of heating a liquid above the
For specific hazard statements, see Section 8.
equilibrium boiling temperature for a particular applied pres-
1.5 This international standard was developed in accor-
sure.
dance with internationally recognized principles on standard-
ization established in the Decision on Principles for the 3.3 Symbols:
Development of International Standards, Guides and Recom-
A, B, C = Antoine vapor pressure equation constants (log ,
mendations issued by the World Trade Organization Technical
kPa, K) for the Antoine vapor pressure equation:
Barriers to Trade (TBT) Committee.
log P = A − B /(T + C).
2. Referenced Documents
P = vapor pressure, kPa.
T = absolute temperature, K.
2.1 ASTM Standards:
D1193 Specification for Reagent Water
4. Summary of Test Method
This test method is under the jurisdiction of ASTM Committee E37 on Thermal 4.1 A specimen is charged to the ebulliometer boiler. The
Measurements and is the direct responsibility of Subcommittee E37.01 on Calo-
ebulliometer is connected to a manostat, and coolant is
rimetry and Mass Loss.
circulated through the ebulliometer condenser. The manostat is
Current edition approved April 1, 2018. Published May 2018. Originally
set at a low pressure, and the specimen is heated to the boiling
approved in 1995. Last previous edition approved in 2012 as E1719 – 12. DOI:
10.1520/E1719-12R18.
temperature. The boiling temperature and manostat pressure
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
are recorded upon reaching a steady-state, and the manostat
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
pressure is raised to a higher value. A suitable number (usually
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. five or more) of boiling temperature points are recorded at
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E1719 − 12 (2018)
successively higher controlled pressures. The pressure-
temperature data are fitted to the Antoine vapor pressure
equation. Vapor pressure values required for specific reports
are then computed from the derived equation.
4.2 The capability of the entire apparatus (ebulliometer,
thermometer, manostat, etc.) is checked periodically by the
procedure described in Annex A1. This procedure consists of
measuring the boiling temperature data for a pure reference
substance such as water and comparing the derived vapor
pressure data to the known reference values.
5. Significance and Use
5.1 Vapor pressure is a fundamental thermodynamic prop-
erty of a liquid. Vapor pressure and boiling temperature data
are required for material safety data sheets (MSDS), the
estimation of volatile organic compounds (VOC), and other
needs related to product safety. Vapor pressures are important
for prediction of the transport of a chemical in the environ-
ment; see Test Method E1194.
6. Interferences
6.1 This test method is limited to materials that are ther-
mally stable over the measurement temperature range. Boiling
temperatures that drift monotonically (not cyclically) up or
down and specimen discoloration and smoking are indications
of thermal instability due to decomposition or polymerization.
See Test Method D2879 (9.3 and Note 8 therein). Vapor
pressure data may be measured at temperatures below the
initial decomposition or polymerization temperature; see 9.7
and 10.2.
6.2 The test method is limited to materials that boil
smoothly under the operation conditions of the ebulliometer.
Materials that “bump” continually, boil erratically, or eject
material through the condenser are not suitable for study by
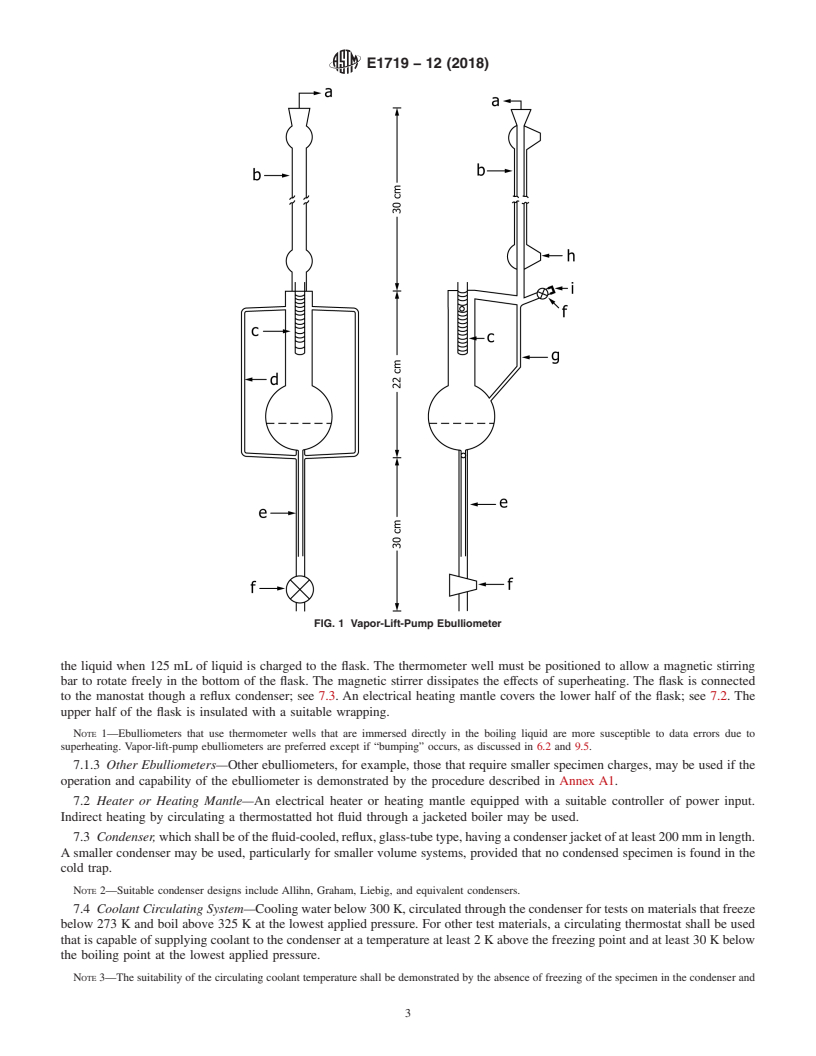
FIG. 1 Vapor-Lift-Pump Ebulliometer
this test method.
7. Apparatus
7.1 Ebulliometer —A vapor-lift-pump, stirred-flask, or
septum port and stopcock (f and i) where materials may be
equivalent type of ebulliometer.
charged to the apparatus. Except for the condenser, septum
7.1.1 For Example, a Vapor-Lift-Pump Ebulliometer —Fig.
port, and stopcock, the entire ebulliometer is insulated with a
1 shows the dimensions for an example twin-arm ebulliometer,
suitable case or wrapping. A window should be left to observe
which is a one-stage, total-reflux boiler equipped with a
the smoothness of boiling and the return rate (drop rate) of
vapor-lift pump to spray slugs of equilibrated liquid and vapor
condensed vapor into the 125-mL boiler return reservoir.
on a thermometer well. The boiler (e), which is constructed
7.1.1.1 For example, a Swietoslawski-type ebulliometer
from concentric pieces of 200-mm glass tubing (5 and 10-mm
may be used instead.
outside diameter), has powdered glass fused to the heated
7.1.2 For example, a Stirred-Flask Ebulliometer, Fig. 2
surface to promote smooth boiling. The boiler is wrapped with
shows an example of a stirred-flask ebulliometer, which is a
an electrical heater. Twin vapor-lift pumps (d-constructed of
one-stage, total-reflux boiler equipped with a magnetic stirrer
270-mm lengths of 5-mm outside diameter glass tubing) spray
to circulate the boiling liquid past a thermometer well which is
liquid and vapor slugs on a 100-mm thermometer well (c) that
immersed in the liquid. The boiler is a 250-mL, round-
is wrapped with a glass spiral to promote thermal equilibration.
bottomed, single-neck boiling flask modified with a 7-mm
The vapor-lift pumps dissipate the effects of superheating. The
inside diameter thermometer well positioned diagonally toward
ebulliometer is connected to the manostat through a 200-mm
the bottom of the flask. The bottom half of the boiler has
reflux condenser (b); see 7.3. The side view in Fig. 1 shows a
powdered glass fused to the inner surface to promote smooth
An ebulliometer can be assembled from readily available lab glassware.
Olson, J. D., Journal of Chemical Engineering Data, Vol 26, 1981, pp. 58–64.
Malanowski, S., Fluid Phase Equilibria, Vol 8, 1982, pp. 197–219.
E1719 − 12 (2018)
7.4 Coolant Circulating System—Cooling water below 300
K, circulated through the condenser for tests on materials that
freeze below 273 K and boil above 325 K at the lowest applied
pressure. For other test materials, a circulating thermostat shall
be used that is capable of supplying coolant to the condenser at
a temperature at least 2 K above the freezing point and at least
30 K below the boiling point at the lowest applied pressure.
NOTE 3—The suitability of the circulating coolant temperature shall be
demonstrated by the absence of freezing of the specimen in the condenser
and the absence of specimen in the cold traps at the conclusion of the test.
7.5 Cold Trap, capable of freezing or condensing the test
material, connected in series to the condenser. Ice plus water,
dry ice plus solvent, or liquid nitrogen may be used as the cold
trap coolant, depending on the characteristics of the test
material.
7.6 Temperature Measuring Device—Liquid-in-glass ther-
mometers accurate to 0.1 K (after calibration and immersion
corrections), or any other thermometric device of equal or
better accuracy. See Specification E1.
7.7 Thermometer Well Fluid—A low-volatility, thermally
inert fluid such as silicone oil or glycerin, charged to the
FIG. 2 Stirred-Flask Ebulliometer
thermometer well of the ebulliometer. The amount of fluid
added should be such that the fluid level in the thermometer
well is not above the flask boundary when the ebulliometer is
boiling. The thermometer well is positioned to have a length
at the measurement temperature.
of at least 20 mm below the surface of the liquid when 125 mL
7.8 Pressure Regulating System—A manostat, capable of
of liquid is charged to the flask. The thermometer well must be
maintaining the pressure of the system constant within 60.07
positioned to allow a magnetic stirring bar to rotate freely in
kPa (60.5 torr). Connect the pressure regulating system to the
the bottom of the flask. The magnetic stirrer dissipates the
exit of the cold trap. A T-connection from the pressure
effects of superheating. The flask is connected to the manostat
regulating system near the exit of the cold trap should be used
though a reflux condenser; see 7.3. An electrical heating mantle
to connect the manometer. A ballast volume may be used to
covers the lower half of the flask; see 7.2. The upper half of the
dampen pressure fluctuations.
flask is insulated with a suitable wrapping.
7.9 Pressure Measuring System—A manometer, capable of
NOTE 1—Ebulliometers that use thermometer wells that are immersed
measuring absolute pressure with an accuracy of 60.07 kPa
directly in the boiling liquid are more susceptible to data errors due to
superheating. Vapor-lift-pump ebulliometers are preferred except if (60.5 torr).
“bumping” occurs, as discussed in 6.2 and 9.5.
7.9.1 A comparative ebulliometer may be used to measure
7.1.3 Other Ebulliometers—Other ebulliometers, for pressure. The comparative ebulliometer is connected to the
example, those that require smaller specimen charges, may be same pressure-controlled atmosphere as the test ebulliometer
used if the operation and capability of the ebulliometer is and contains a reference fluid (for example, distilled water).
demonstrated by the procedure described in Annex A1. The observed boiling temperature in the comparative ebulli-
ometer is used to compute the applied pressure from the known
7.2 Heater or Heating Mantle—An electrical heater or
vapor pressure-temperature relationship of the reference fluid.
heating mantle equipped with a suitable controller of power
input. Indirect heating by circulating a thermostatted hot fluid
7.10 Software, to perform multiple linear regression analy-
through a jacketed boiler may be used.
sis on three variables.
7.3 Condenser, which shall be of the fluid-cooled, reflux,
8. Safety Precautions
glass-tube type, having a condenser jacket of at least 200 mm
in length. A smaller condenser may be used, particularly for
8.1 There shall be adequate provisions for the retention and
smaller volume systems, provided that no condensed specimen
disposal of spilled mercury if mercury-containing
is found in the cold trap.
thermometers, pressure measurement, or controlling devices
are used.
NOTE 2—Suitable condenser designs include Allihn, Graham, Liebig,
and equivalent condensers.
8.2 Vapor pressure reference materials (Annex A1) and
many test materials and cold trap fluids will burn. Adequate
precautions shall be taken to eliminate ignition sources and
The stirred-flask ebulliometer shown in Fig. 2 (with the inner boiling surface
provide ventilation to remove flammable vapors that are
coated with powdered glass) is available from Lab Glass, Inc., P.O. Box 5067, Fort
Henry Drive, Kingsport, TN 37663. generated during operation of the ebulliometer.
E1719 − 12 (2018)
8.3 Adequate precautions shall be taken to protect the plateau” is reached at which the observed temperature is
operator in case debris is scattered by an implosion of glass independent of the heater power.
apparatus under vacuum. 9.6.2 At steady-state, the boiling temperature should be
independent of the heater power (applied voltage) over a
9. Procedure modest range (approximately 5 V for an ebulliometer with a
variable transformer).
9.1 Start with clean, dry apparatus. Verify the operation and
9.7 Discontinue the test if the specimen begins to decom-
capability of the apparatus as described in Annex A1 for a new
pose or polymerize. Decomposition may be indicated by a
ebulliometer setup or an ebulliometer setup that has not been
decreasing boiling point temperature, smoking or extreme
used recently.
discoloration of the specimen, or failure to reach a steady-state.
9.2 Charge a specimen of appropriate volume to the ebulli-
Polymerization of the specimen usually causes the temperature
ometer boiler. Charge 75 6 1 mL for the vapor-lift ebulliom-
to continue to increase instead of reaching a steady-state.
eter (Fig. 1). Close all stopcocks on the vapor-lift ebull
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E1719 − 12 E1719 − 12 (Reapproved 2018)
Standard Test Method for
Vapor Pressure of Liquids by Ebulliometry
This standard is issued under the fixed designation E1719; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method describes procedures for determination of the vapor pressure of liquids by ebulliometry (boiling point
measurements). It is applicable to pure liquids and azeotropes that have an atmospheric boiling point between 285 and 575 K and
that can be condensed completely and returned to the ebulliometer boiler, that is, all materials must be condensable at total reflux.
Liquid mixtures may be studied if they do not contain non-condensable components. Liquid mixtures that contain trace amounts
of volatile but completely condensable components may also be studied, but they will produce vapor pressure data of greater
uncertainty. Boiling point temperatures are measured at applied pressures of 1.0 to 100 kPa (7.5 to 760 torr).
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 There is no ISO equivalent to this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use. For specific hazard statements, see Section 8.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D1193 Specification for Reagent Water
D2879 Test Method for Vapor Pressure-Temperature Relationship and Initial Decomposition Temperature of Liquids by
Isoteniscope
E1 Specification for ASTM Liquid-in-Glass Thermometers
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E1142 Terminology Relating to Thermophysical Properties
E1194 Test Method for Vapor Pressure
E1970 Practice for Statistical Treatment of Thermoanalytical Data
3. Terminology
3.1 Definitions:
3.1.1 The following terms are applicable to this test method and can be found in Terminology E1142; boiling temperature and
vapor pressure.
3.1.2 For definitions of other terms used in this test method, refer to Terminology E1142.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 ebulliometer—a one-stage, total-reflux boiler designed to minimize superheating of the boiling liquid.
3.2.2 manostat—a device for maintaining constant vacuum or pressure.
3.2.3 superheating—the act of heating a liquid above the equilibrium boiling temperature for a particular applied pressure.
This test method is under the jurisdiction of ASTM Committee E37 on Thermal Measurements and is the direct responsibility of Subcommittee E37.01 on Calorimetry
and Mass Loss.
Current edition approved April 1, 2012April 1, 2018. Published July 2012May 2018. Originally approved in 1995. Last previous edition approved in 20052012 as
E1719 – 05.E1719 – 12. DOI: 10.1520/E1719-12.10.1520/E1719-12R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E1719 − 12 (2018)
3.3 Symbols:
A, B, C = Antoine vapor pressure equation constants (log , kPa, K) for the Antoine vapor pressure equation: log P = A − B
10 10
/(T + C).
P = vapor pressure, kPa.
T = absolute temperature, K.
4. Summary of Test Method
4.1 A specimen is charged to the ebulliometer boiler. The ebulliometer is connected to a manostat, and coolant is circulated
through the ebulliometer condenser. The manostat is set at a low pressure, and the specimen is heated to the boiling temperature.
The boiling temperature and manostat pressure are recorded upon reaching a steady-state, and the manostat pressure is raised to
a higher value. A suitable number (usually five or more) of boiling temperature points are recorded at successively higher
controlled pressures. The pressure-temperature data are fitted to the Antoine vapor pressure equation. Vapor pressure values
required for specific reports are then computed from the derived equation.
4.2 The capability of the entire apparatus (ebulliometer, thermometer, manostat, etc.) is checked periodically by the procedure
described in Annex A1. This procedure consists of measuring the boiling temperature data for a pure reference substance such as
water and comparing the derived vapor pressure data to the known reference values.
5. Significance and Use
5.1 Vapor pressure is a fundamental thermodynamic property of a liquid. Vapor pressure and boiling temperature data are
required for material safety data sheets (MSDS), the estimation of volatile organic compounds (VOC), and other needs related to
product safety. Vapor pressures are important for prediction of the transport of a chemical in the environment; see Test Method
E1194.
6. Interferences
6.1 This test method is limited to materials that are thermally stable over the measurement temperature range. Boiling
temperatures that drift monotonically (not cyclically) up or down and specimen discoloration and smoking are indications of
thermal instability due to decomposition or polymerization. See Test Method D2879 (9.3 and Note 8 therein). Vapor pressure data
may be measured at temperatures below the initial decomposition or polymerization temperature; see 9.7 and 10.2.
6.2 The test method is limited to materials that boil smoothly under the operation conditions of the ebulliometer. Materials that“
bump”that “bump” continually, boil erratically, or eject material through the condenser are not suitable for study by this test
method.
7. Apparatus
7.1 Ebulliometer —A vapor-lift-pump, stirred-flask, or equivalent type of ebulliometer.
7.1.1 For Example, a Vapor-Lift-Pump Ebulliometer —Fig. 1 shows the dimensions for an example twin-arm ebulliometer,
which is a one-stage, total-reflux boiler equipped with a vapor-lift pump to spray slugs of equilibrated liquid and vapor on a
thermometer well. The boiler (e), which is constructed from concentric pieces of 200-mm glass tubing (5 and 10-mm outside
diameter), has powdered glass fused to the heated surface to promote smooth boiling. The boiler is wrapped with an electrical
heater. Twin vapor-lift pumps (d-constructed of 270-mm lengths of 5-mm outside diameter glass tubing) spray liquid and vapor
slugs on a 100-mm thermometer well (c) that is wrapped with a glass spiral to promote thermal equilibration. The vapor-lift pumps
dissipate the effects of superheating. The ebulliometer is connected to the manostat through a 200-mm reflux condenser (b); see
7.3. The side view in Fig. 1 shows a septum port and stopcock (f and i) where materials may be charged to the apparatus. Except
for the condenser, septum port, and stopcock, the entire ebulliometer is insulated with a suitable case or wrapping. A window
should be left to observe the smoothness of boiling and the return rate (drop rate) of condensed vapor into the 125-mL boiler return
reservoir.
7.1.1.1 For example, a Swietoslawski-type ebulliometer may be used instead.
7.1.2 For example, a Stirred-Flask Ebulliometer,Fig. 2 shows an example of a stirred-flask ebulliometer, which is a one-stage,
total-reflux boiler equipped with a magnetic stirrer to circulate the boiling liquid past a thermometer well which is immersed in
the liquid. The boiler is a 250-mL, round-bottomed, single-neck boiling flask modified with a 7-mm inside diameter thermometer
well positioned diagonally toward the bottom of the flask. The bottom half of the boiler has powdered glass fused to the inner
surface to promote smooth boiling. The thermometer well is positioned to have a length of at least 20 mm below the surface of
An ebulliometer can be assembled from readily available lab glassware.
Olson, J.D., Journal of Chemical Engineering Data, Vol 26, 1981, pp. 58–64. Olson, J. D., Journal of Chemical Engineering Data, Vol 26, 1981, pp. 58–64.
Malanowski, S., Fluid Phase Equilibria, Vol 8, 1982, pp. 197–219. Malanowski, S., Fluid Phase Equilibria, Vol 8, 1982, pp. 197–219.
The stirred-flask ebulliometer shown in Fig. 2 (with the inner boiling surface coated with powdered glass) is available from Lab Glass, Inc., P.O. Box 5067, Fort Henry
Drive, Kingsport, TN 37663.
E1719 − 12 (2018)
FIG. 1 Vapor-Lift-Pump Ebulliometer
the liquid when 125 mL of liquid is charged to the flask. The thermometer well must be positioned to allow a magnetic stirring
bar to rotate freely in the bottom of the flask. The magnetic stirrer dissipates the effects of superheating. The flask is connected
to the manostat though a reflux condenser; see 7.3. An electrical heating mantle covers the lower half of the flask; see 7.2. The
upper half of the flask is insulated with a suitable wrapping.
NOTE 1—Ebulliometers that use thermometer wells that are immersed directly in the boiling liquid are more susceptible to data errors due to
superheating. Vapor-lift-pump ebulliometers are preferred except if “bumping” occurs, as discussed in 6.2 and 9.5.
7.1.3 Other Ebulliometers—Other ebulliometers, for example, those that require smaller specimen charges, may be used if the
operation and capability of the ebulliometer is demonstrated by the procedure described in Annex A1.
7.2 Heater or Heating Mantle—An electrical heater or heating mantle equipped with a suitable controller of power input.
Indirect heating by circulating a thermostatted hot fluid through a jacketed boiler may be used.
7.3 Condenser, which shall be of the fluid-cooled, reflux, glass-tube type, having a condenser jacket of at least 200 mm in length.
A smaller condenser may be used, particularly for smaller volume systems, provided that no condensed specimen is found in the
cold trap.
NOTE 2—Suitable condenser designs include Allihn, Graham, Liebig, and equivalent condensers.
7.4 Coolant Circulating System—Cooling water below 300 K, circulated through the condenser for tests on materials that freeze
below 273 K and boil above 325 K at the lowest applied pressure. For other test materials, a circulating thermostat shall be used
that is capable of supplying coolant to the condenser at a temperature at least 2 K above the freezing point and at least 30 K below
the boiling point at the lowest applied pressure.
NOTE 3—The suitability of the circulating coolant temperature shall be demonstrated by the absence of freezing of the specimen in the condenser and
E1719 − 12 (2018)
FIG. 2 Stirred-Flask Ebulliometer
the absence of specimen in the cold traps at the conclusion of the test.
7.5 Cold Trap, capable of freezing or condensing the test material, connected in series to the condenser. Ice plus water, dry ice
plus solvent, or liquid nitrogen may be used as the cold trap coolant, depending on the characteristics of the test material.
7.6 Temperature Measuring Device—Liquid-in-glass thermometers accurate to 0.1 K (after calibration and immersion
corrections), or any other thermometric device of equal or better accuracy. See Specification E1.
7.7 Thermometer Well Fluid—A low-volatility, thermally inert fluid such as silicone oil or glycerin, charged to the thermometer
well of the ebulliometer. The amount of fluid added should be such that the fluid level in the thermometer well is not above the
flask boundary when the ebulliometer is at the measurement temperature.
7.8 Pressure Regulating System—A manostat, capable of maintaining the pressure of the system constant within 60.07 kPa
(60.5 torr). Connect the pressure regulating system to the exit of the cold trap. A T-connection from the pressure regulating system
near the exit of the cold trap should be used to connect the manometer. A ballast volume may be used to dampen pressure
fluctuations.
7.9 Pressure Measuring System—A manometer, capable of measuring absolute pressure with an accuracy of 60.07 kPa (60.5
torr).
7.9.1 A comparative ebulliometer may be used to measure pressure. The comparative ebulliometer is connected to the same
pressure-controlled atmosphere as the test ebulliometer and contains a reference fluid (for example, distilled water). The observed
boiling temperature in the comparative ebulliometer is used to compute the applied pressure from the known vapor
pressure-temperature relationship of the reference fluid.
7.10 Software, to perform multiple linear regression analysis on three variables.
8. Safety Precautions
8.1 There shall be adequate provisions for the retention and disposal of spilled mercury if mercury-containing thermometers,
pressure measurement, or controlling devices are used.
8.2 Vapor pressure reference materials (Annex A1) and many test materials and cold trap fluids will burn. Adequate precautions
shall be taken to eliminate ignition sources and provide ventilation to remove flammable vapors that are generated during operation
of the ebulliometer.
8.3 Adequate precautions shall be taken to protect the operator in case debris is scattered by an implosion of glass apparatus
under vacuum.
9. Procedure
9.1 Start with clean, dry apparatus. Verify the operation and capability of the apparatus as described in Annex A1 for a new
ebulliometer setup or an ebulliometer setup that has not been used recently.
E1719 − 12 (2018)
9.2 Charge a specimen of appropriate volume to the ebulliometer boiler. Charge 75 6 1 mL for the vapor-lift ebulliometer (Fig.
1). Close all stopcocks on the vapor-lift ebulliometer. Charge 125 6 1 mL for the stirred-flask ebulliometer (Fig. 2). Add a magnetic
stirring bar to the stirred-flask ebulliometer. Conn
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.