ASTM E1194-17
(Test Method)Standard Test Method for Vapor Pressure
Standard Test Method for Vapor Pressure
SIGNIFICANCE AND USE
5.1 Vapor pressure values can be used to predict volatilization rates (5). Vapor pressures, along with vapor-liquid partition coefficients (Henry's Law constant) are used to predict volatilization rates from liquids such as water. These values are thus particularly important for the prediction of the transport of a chemical in the environment (6).
SCOPE
1.1 This test method describes procedures for measuring the vapor pressure of pure liquid or solid compounds. No single technique is able to measure vapor pressures from 1 × 10−11 to 100 kPa (approximately 10−10 to 760 torr). The subject of this standard is gas saturation which is capable of measuring vapor pressures from 1 × 10–11 to 1 kPa (approximately 10–10 to 10 torr). Other methods, such as isoteniscope and differential scanning calorimetry (DSC) are suitable for measuring vapor pressures above 0.1 kPa An isoteniscope (standard) procedure for measuring vapor pressures of liquids from 1 × 10−1 to 100 kPa (approximately 1 to 760 torr) is available in Test Method D2879. A DSC (standard) procedure for measuring vapor pressures from 2 × 10−1 to 100 kPa (approximately 1 to 760 torr) is available in Test Method E1782. A gas-saturation procedure for measuring vapor pressures from 1 × 10−11 to 1 kPa (approximately 10−10 to 10 torr) is presented in this test method. All procedures are subjects of U.S. Environmental Protection Agency Test Guidelines.
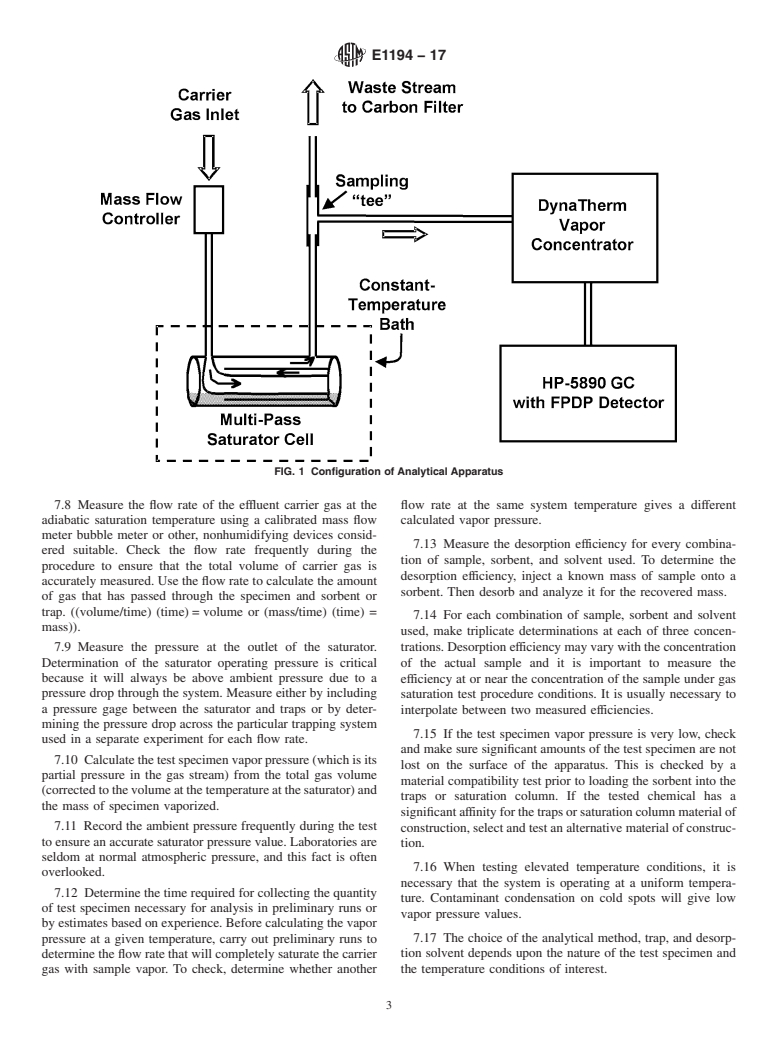
1.2 The gas saturation method is very useful for providing vapor pressure data at normal environmental temperatures (–40 to +60°C). At least three temperature values should be studied to allow definition of a vapor pressure-temperature correlation. Values determined should be based on temperature selections such that a measurement is made at 25°C (as recommended by IUPAC) (1),2 a value can be interpolated for 25°C, or a value can be reliably extrapolated for 25°C. Extrapolation to 25°C should be avoided if the temperature range tested includes a value at which a phase change occurs. Extrapolation to 25°C over a range larger than 10°C should also be avoided. If possible, the temperatures investigated should be above and below 25°C to avoid extrapolation altogether. The gas saturation method was selected because of its extended range, simplicity, and general applicability (2). Examples of results produced by the gas-saturation procedure during an interlaboratory evaluation are given in Table 1. These data have been taken from Reference (3). (A) Sr is the estimated standard deviation within laboratories, that is, an average of the repeatability found in the separate laboratories.(B) SR is the square root of the component of variance between laboratories.(C) SR is the between-laboratory estimate of precision.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E1194 − 17
Standard Test Method for
1
Vapor Pressure
This standard is issued under the fixed designation E1194; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ratory evaluation are given in Table 1. These data have been
taken from Reference (3).
1.1 Thistestmethoddescribesproceduresformeasuringthe
vapor pressure of pure liquid or solid compounds. No single 1.3 The values stated in SI units are to be regarded as
−11
technique is able to measure vapor pressures from 1×10 to standard. No other units of measurement are included in this
−10
100 kPa (approximately 10 to 760 torr). The subject of this standard.
standard is gas saturation which is capable of measuring vapor
1.4 This standard does not purport to address all of the
–11 –10
pressures from 1×10 to 1 kPa (approximately 10 to 10
safety problems, if any, associated with its use. It is the
torr). Other methods, such as isoteniscope and differential
responsibility of the user of this standard to establish appro-
scanning calorimetry (DSC) are suitable for measuring vapor
priate safety and health practices and determine the applica-
pressures above 0.1 kPaAn isoteniscope (standard) procedure
bility of regulatory limitations prior to use.
−1
for measuring vapor pressures of liquids from 1×10 to 100
kPa (approximately 1 to 760 torr) is available in Test Method
2. Referenced Documents
D2879. A DSC (standard) procedure for measuring vapor 3
2.1 ASTM Standards:
−1
pressures from 2×10 to 100 kPa (approximately 1 to 760
D2879Test Method for Vapor Pressure-Temperature Rela-
torr) is available in Test Method E1782. A gas-saturation
tionship and Initial Decomposition Temperature of Liq-
−11
procedure for measuring vapor pressures from 1×10 to 1
uids by Isoteniscope
−10
kPa (approximately 10 to 10 torr) is presented in this test
E691Practice for Conducting an Interlaboratory Study to
method. All procedures are subjects of U.S. Environmental
Determine the Precision of a Test Method
Protection Agency Test Guidelines.
E1782Test Method for Determining Vapor Pressure by
1.2 The gas saturation method is very useful for providing
Thermal Analysis
vaporpressuredataatnormalenvironmentaltemperatures(–40
2.2 U.S. Environmental Protection Agency Test Guidelines:
to +60°C).At least three temperature values should be studied
Toxic Substances Control Act Test Guidelines; Final Rules,
4
toallowdefinitionofavaporpressure-temperaturecorrelation.
Vapor Pressure
Values determined should be based on temperature selections
such that a measurement is made at 25°C (as recommended by 3. Terminology Definition
2
IUPAC) (1), a value can be interpolated for 25°C, or a value
3.1 vaporpressure—ameasureofthevolatilityinunitsofor
can be reliably extrapolated for 25°C. Extrapolation to 25°C 2
equivalenttokg/m (pascal)ofasubstanceinequilibriumwith
should be avoided if the temperature range tested includes a
the pure liquid or solid of that same substance at a given
value at which a phase change occurs. Extrapolation to 25°C
temperature (4).
over a range larger than 10°C should also be avoided. If
possible, the temperatures investigated should be above and
4. Summary of Gas-Saturation Method
below 25°C to avoid extrapolation altogether. The gas satura-
4.1 Pressures less than 1.33 kPa may be measured using the
tion method was selected because of its extended range,
gas-saturation procedure (4).
simplicity, and general applicability (2). Examples of results
4.2 Inthistestmethod,aninertcarriergas(forexampleN )
produced by the gas-saturation procedure during an interlabo- 2
is passed through a sufficient amount of compound to maintain
saturation for the duration of the test. The compound may be
1
This test method is under the jurisdiction of ASTM Committee E50 on
coatedontoaninertsupport(forexampleglassbeads)oritmay
Environmental Assessment, Risk Management and Corrective Actionand is the
direct responsibility of Subcommittee E50.47 on Biological Effects and Environ-
mental Fate.
3
Current edition approved March 1, 2017. Published March 2017. Originally For referenced ASTM standards, visit the ASTM website, www.astm.org, or
approved in 1987. Last previous edition approved in 2007 as E1194 which was contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
withdrawn March 2013 and reinstated in March 2017. DOI: 10.1520/E1194-17. Standards volume information, refer to the standard’s Document Summary page on
2
T
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.