ASTM D5453-00
(Test Method)Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Motor Fuels and Oils by Ultraviolet Fluorescence
Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Motor Fuels and Oils by Ultraviolet Fluorescence
SCOPE
1.1 This test method covers the determination of total sulfur in liquid hydrocarbons, boiling in the range from approximately 25 to 400oC, with viscosities between approximately 0.2 and 20 cSt (mm 2/S) at room temperature.
1.2 Three separate inter-laboratory studies (ILS) on precision have determined that this test method is applicable to naphthas, distillates, oils, and motor fuels: such as gasoline, oxygen enriched gasoline (M-85, RFG), diesel, biodiesel and jet fuel. Samples containing 1.0 to 8000 mg/kg total sulfur can be analyzed (Note 1).
Note 1-Estimates of the pooled limit of quantification (PLOQ) for each of the three precision studies were calculated. Values ranged between less than 1.0 and less than 5.0 mg/kg (see Section 8 and 14.1).
1.3 This test method is applicable for total sulfur determination in liquid hydrocarbons containing less than 0.35 % (m/m) halogen(s).
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. See 3.1, 6.3, 6.4, 8.1 and Section 7.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 5453 – 00 An American National Standard
Standard Test Method for
Determination of Total Sulfur in Light Hydrocarbons, Motor
Fuels and Oils by Ultraviolet Fluorescence
This standard is issued under the fixed designation D 5453; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 6299 Practice for Applying Statistical Quality Assurance
Techniques to Evaluate Analytical Measurement System
1.1 This test method covers the determination of total sulfur
Performance
in liquid hydrocarbons, boiling in the range from approxi-
mately 25 to 400°C, with viscosities between approximately
3. Summary of Test Method
0.2 and 20 cSt (mm /S) at room temperature.
3.1 A hydrocarbon sample is either directly injected or
1.2 Three separate inter-laboratory studies (ILS) on preci-
placed in a sample boat. The sample or boat, or both, is inserted
sion have determined that this test method is applicable to
into a high temperature combustion tube where the sulfur is
naphthas, distillates, oils, and motor fuels: such as gasoline,
oxidized to sulfur dioxide (SO ) in an oxygen rich atmosphere.
oxygen enriched gasoline (M-85, RFG), diesel, biodiesel and
Water produced during the sample combustion is removed and
jet fuel. Samples containing 1.0 to 8000 mg/kg total sulfur can
the sample combustion gases are next exposed to ultraviolet
be analyzed (Note 1).
(UV) light. The SO absorbs the energy from the UV light and
NOTE 1—Estimates of the pooled limit of quantification (PLOQ) for
is converted to excited sulfur dioxide (SO *). The fluorescence
each of the three precision studies were calculated. Values ranged between
emitted from the excited SO * as it returns to a stable state SO
2 2
less than 1.0 and less than 5.0 mg/kg (see Section 8 and 14.1).
is detected by a photomultiplier tube and the resulting signal is
1.3 This test method is applicable for total sulfur determi-
a measure of the sulfur contained in the sample. Warning—
nation in liquid hydrocarbons containing less than 0.35 %
Exposure to excessive quantities of ultraviolet (UV) light is
(m/m) halogen(s).
injurious to health. The operator must avoid exposing any part
1.4 The values stated in SI units are to be regarded as
of their person, especially their eyes, not only to direct UV
standard.
light but also to secondary or scattered radiation that is present.
1.5 This standard does not purport to address all of the
4. Significance and Use
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4.1 Some process catalysts used in petroleum and chemical
priate safety and health practices and determine the applica-
refining can be poisoned when trace amounts of sulfur bearing
bility of regulatory limitations prior to use. See 3.1, 6.3, 6.4,
materials are contained in the feedstocks. This test method can
8.1 and Section 7.
be used to determine sulfur in process feeds sulfur in finished
products, and can also be used for purposes of regulatory
2. Referenced Documents
control.
2.1 ASTM Standards:
5. Apparatus
D 1298 Test Method for Density, Relative Density (Specific
Gravity), or API Gravity of Crude Petroleum and Liquid
5.1 Furnace—An electric furnace held at a temperature
Petroleum Products by Hydrometer Method
(1075 6 25°C) sufficient to pyrolyze all of the sample and
D 4052 Test Method for Density and Relative Density of
oxidize sulfur to SO .
Liquids by Digital Density Meter
5.2 Combustion Tube—A quartz combustion tube con-
D 4057 Practice for Manual Sampling of Petroleum and
structed to allow the direct injection of the sample into the
Petroleum Products
heated oxidation zone of the furnace or constructed so that the
D 4177 Practice for Automatic Sampling of Petroleum and
inlet end of the tube is large enough to accommodate a quartz
Petroleum Products
sample boat. The combustion tube must have side arms for the
introduction of oxygen and carrier gas. The oxidation section
shall be large enough (see Fig. 1 and Fig. 2) to ensure complete
This test method is under the jurisdiction of ASTM Committee D-02 on
Petroleum Products and Lubricantsand is the direct responsibility of Subcommittee
D02.030C on Electrometric Methods.
Current edition approved Jan. 10, 2000. Published March 2000. Originally
published as D – 93. Last previous edition D – 93. Annual Book of ASTM Standards, Vol 05.04.
2 5
Annual Book of ASTM Standards, Vol 05.01. Apparatus manufactured in several variations by Antek Instruments, Inc.,
Annual Book of ASTM Standards, Vol 05.02. Houston, TX has been found suitable for this purpose.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
D 5453
FIG. 1 Direct Inject Quartz Pyrolysis Tube
FIG. 2 Boat Inlet
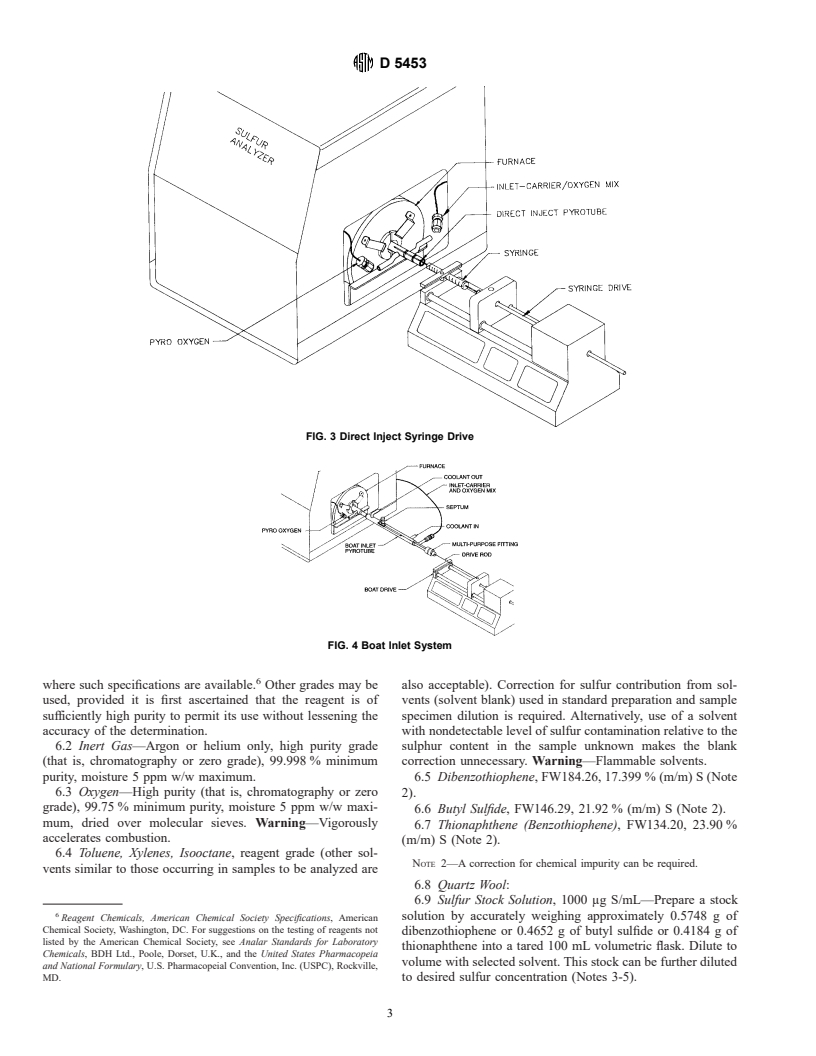
combustion of the sample. Fig. 1 and Fig. 2 depict conven- 5.7.2 Boat Inlet System—An extended combustion tube
tional combustion tubes. Other configurations are acceptable if
provides a seal to the inlet of the oxidation area and is swept by
precision is not degraded.
a carrier gas. The system provides an area to position the
5.3 Flow Control—The apparatus must be equipped with
sample carrying mechanism (boat) at a retracted position
flow controllers capable of maintaining a constant supply of
removed from the furnace. The boat drive mechanism will
oxygen and carrier gas.
fully insert the boat into the hottest section of the furnace inlet.
5.4 Drier Tube—The apparatus must be equipped with a
The sample boats and combustion tube are constructed of
mechanism for the removal of water vapor. The oxidation
quartz. The combustion tube provides a cooling jacket for the
reaction produces water vapor which must be eliminated prior
area in which the retracted boat rests awaiting sample intro-
to measurement by the detector. This can be accomplished with
duction from a microlitre syringe. A drive mechanism which
a membrane drying tube, or a permeation dryer, that utilizes a
advances and withdraws the sample boat into and out of the
selective capillary action for water removal.
furnace at a controlled and repeatable rate is required. See
5.5 UV Fluorescence Detector—A qualitative and quantita-
example, Fig. 4.
tive detector capable of measuring light emitted from the
5.8 Refrigerated Circulator—An adjustable apparatus ca-
fluorescence of sulfur dioxide by UV light.
pable of delivering a coolant material at a constant temperature
5.6 Microlitre Syringe—A microlitre syringe capable of
as low as 4°C could be required when using the boat inlet
accurately delivering 5 to 20-μL quantities. The needle shall be
injection method (optional).
50 mm (6 5 mm) long.
5.7 Sample Inlet System—Either of two types of sample
5.9 Strip Chart Recorder, (optional).
inlet systems can be used.
5.10 Balance, with a precision of 6 0.01 mg (optional).
5.7.1 Direct Injection—A direct injection inlet system must
be capable of allowing the quantitative delivery of the material
6. Reagents
to be analyzed into an inlet carrier stream which directs the
6.1 Purity of Reagents—Reagent grade chemicals shall be
sample into the oxidation zone at a controlled and repeatable
used in tests. Unless otherwise indicated, it is intended that all
rate. A syringe drive mechanism which discharges the sample
reagents shall conform to the specifications of the Committee
from the microlitre syringe at a rate of approximately 1 μL/s is
required. See example, Fig. 3. on Analytical Reagents of the American Chemical Society,
D 5453
FIG. 3 Direct Inject Syringe Drive
FIG. 4 Boat Inlet System
where such specifications are available. Other grades may be also acceptable). Correction for sulfur contribution from sol-
used, provided it is first ascertained that the reagent is of vents (solvent blank) used in standard preparation and sample
sufficiently high purity to permit its use without lessening the specimen dilution is required. Alternatively, use of a solvent
accuracy of the determination. with nondetectable level of sulfur contamination relative to the
6.2 Inert Gas—Argon or helium only, high purity grade sulphur content in the sample unknown makes the blank
(that is, chromatography or zero grade), 99.998 % minimum correction unnecessary. Warning—Flammable solvents.
purity, moisture 5 ppm w/w maximum.
6.5 Dibenzothiophene, FW184.26, 17.399 % (m/m) S (Note
6.3 Oxygen—High purity (that is, chromatography or zero 2).
grade), 99.75 % minimum purity, moisture 5 ppm w/w maxi-
6.6 Butyl Sulfide, FW146.29, 21.92 % (m/m) S (Note 2).
mum, dried over molecular sieves. Warning—Vigorously
6.7 Thionaphthene (Benzothiophene), FW134.20, 23.90 %
accelerates combustion.
(m/m) S (Note 2).
6.4 Toluene, Xylenes, Isooctane, reagent grade (other sol-
NOTE 2—A correction for chemical impurity can be required.
vents similar to those occurring in samples to be analyzed are
6.8 Quartz Wool:
6.9 Sulfur Stock Solution, 1000 μg S/mL—Prepare a stock
solution by accurately weighing approximately 0.5748 g of
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
dibenzothiophene or 0.4652 g of butyl sulfide or 0.4184 g of
listed by the American Chemical Society, see Analar Standards for Laboratory
thionaphthene into a tared 100 mL volumetric flask. Dilute to
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
volume with selected solvent. This stock can be further diluted
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
MD. to desired sulfur concentration (Notes 3-5).
D 5453
NOTE 3—Working standards should be remixed on a regular basis TABLE 2 Sulfur Standards
depending upon frequency of use and age. Typically, stock solutions have
Curve I Curve II Curve III
a useful life of about 3 months.
Sulfur, ng/μL Sulfur, ng/μL Sulfur, ng/μL
NOTE 4—Calibration standards can be prepared and diluted on a
0.50 5.00 100.00
mass/mass basis when result calculations are adjusted to accommodate
1.00 25.00 500.00
them.
2.50 50.00 1000.00
NOTE 5—Calibration standards from commercial sources can be used if 5.00 100.00
10.00
checked for accuracy and if precision is not degraded.
Injection Size Injection Size Injection Size
6.10 Quality Control (QC) Samples, preferably are portions
10–20 μL 5–10 μL 5 μL
of one or more liquid petroleum materials that are stable and
representative of the samples of interest. These QC samples
can be used to check the validity of the testing process as
10.2 Flush the microlitre syringe several times with the
described in Section 13.
sample prior to analysis. If bubbles are present in the liquid
column, flush the syringe and withdraw a new sample.
7. Hazards
10.3 A sample size recommended for the curve selected
7.1 High temperature is employed in this test method. Extra
from Table 2 must be quantitatively measured prior to injection
care must be exercised when using flammable materials near
into the combustion tube or delivery into the sample boat for
the oxidative pyrolysis furnace.
analysis (Note 6 and Note 7). There are two alternative
techniques available.
8. Sampling
NOTE 6—Injection of a constant or similar sample size for all materials
8.1 Obtain a test unit in accordance with Practice D 4057 or
analyzed in a selected operating range promotes consistent combustion
Practice D 4177. To preserve volatile components which are in
conditions.
some samples, do not uncover samples any longer than
NOTE 7—Injection of 10 μL of the 100 ng/μL standard would establish
necessary. Samples shall be analyzed as soon as possible after
a calibration point equal to 1000 ng or 1.0 μg.
taking from bulk supplies to prevent loss of sulfur or contami-
10.3.1 The volumetric measurement of the injected material
nation due to exposure or contact with sample container.
can be obtained by filling the syringe to the selected level.
Warning—Samples that are collected at temperatures below
Retract the plunger so that air is aspirated and the lower liquid
room temperature can undergo expansion and rupture the
meniscus falls on the 10 % scale mark and record the volume
container. For such samples, do not fill the container to the top;
of liquid in the syringe. After injection, again retract the
leave sufficient air space above the sample to allow room for
plunger so that the lower liquid meniscus falls on the 10 %
expansion.
scale mark and record the volume of liquid in the syringe. The
8.2 If the test unit is not used immediately, then thoroughly
difference between the two volume readings is the volume of
mix in its container prior to taking a test specimen.
sample injected (Note 8).
9. Preparation of Apparatus
NOTE 8—An automatic sampling and injection device can be used in
place of the described manual injection procedure.
9.1 Assemble and leak check apparatus according to manu-
facturer’s instructions.
10.3.2 Fill the syringe as described in 10.3.1. Weigh the
9.2 Adjust the apparatus, depending upon the method of
device before and after injection to determine the amount of
sample introduction, to meet conditions described in Table 1.
sample injected. This procedure can provide greater accuracy
9.3 Adjust the instrument sensitivity and baseline stability
than the volume delivery method, provided a balance with a
and perform instrument blanking procedures following manu-
precision of 60.01 mg is used.
facturer’s guidelines.
10.4 Once the appropriate sample size has been measured
into the microlitre syringe, promptly and quantitatively deliver
10. Calibration and Standardization
the sample into the apparatus. Again, there are two alternative
10.1 Based on anticipated sulfur concentration, select one of
techniques available.
the suggested curves outlined in Table 2. Carefully prepare a
10.4.1 For direct injection, carefully insert the syringe into
series of calibration standards accordingly. Make other volu-
the inlet of the combustion tube and the syringe drive. Allow
metric dilutions of the stock solution to cover the various
time for sample residues to be burned from the needle (Needle
ranges of operation within these calibration curve guidelines.
Blank). Once a stable baseline has reestablished, promptly start
The number of standards used per curve can vary, if equivalent
the analysis. Remove syringe once the apparatus has returned
results are obtained.
to a stable baseline.
10.4.2 For the boat inlet, quantitatively disch
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.