ASTM D6350-98(2003)
(Test Method)Standard Test Method for Mercury Sampling and Analysis in Natural Gas by Atomic Fluorescence Spectroscopy (Withdrawn 2012)
Standard Test Method for Mercury Sampling and Analysis in Natural Gas by Atomic Fluorescence Spectroscopy (Withdrawn 2012)
SIGNIFICANCE AND USE
This test method can be used to determine the total mercury concentration of a natural gas stream down to 0.001 μg/m3. It can be used to assess compliance with environmental regulations, predict possible damage to gas plant equipment, and monitor the efficiency of mercury removal beds.
The preferred sampling method for mercury collection is on supported gold sorbent, which allows the element to be trapped and extracted from the interfering matrix of the gas. Thermal desorption of mercury is performed by raising the temperature of the trap by means of a nichrome wire coiled around it.
Since AFS demonstrates lower detection limits approaching 0.1 pg, this test method avoids difficulties associated with prolonged sampling time. Saturation of the trap with interferants such as hydrogen sulfide (H2S) is avoided. Average sampling can range between 15 to 30 min, or less.
SCOPE
1.1 This test method covers the determination of total mercury in natural gas streams down to 0.001 μg/m3. It includes procedures to both obtaining a representative sample and the atomic fluorescence detection of the analyte. This procedure can be applied for both organic and inorganic mercury compounds.
1.2 Both, inch-pound and SI (metric) units of measurement are used throughout this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to its use.
WITHDRAWN RATIONALE
This test method covers the determination of total mercury in natural gas streams down to 0.001 &mgr;g/m3. It includes procedures to both obtaining a representative sample and the atomic fluorescence detection of the analyte. This procedure can be applied for both organic and inorganic mercury compounds.
Formerly under the jurisdiction of Committee D03 on Gaseous Fuels, this test method was withdrawn in July 2012 in accordance with section 10.5.3.1 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end of the eighth year since the last approval date.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D6350–98 (Reapproved 2003)
Standard Test Method for
Mercury Sampling and Analysis in Natural Gas by Atomic
Fluorescence Spectroscopy
This standard is issued under the fixed designation D6350; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 253.652 nm. Excitation of mercury atoms produces resonance
fluorescencewhichreradiatesattheexcitationwavelength.The
1.1 This test method covers the determination of total
fluorescenceradiationisdetectedbyaphotomultipliertubeand
mercury in natural gas streams down to 0.001 µg/m.It
is directly proportional to the amount of mercury in the cell.
includes procedures to both obtaining a representative sample
The concentration of the element in the original sample is
and the atomic fluorescence detection of the analyte. This
obtained by comparison to freshly prepared standards, which
procedure can be applied for both organic and inorganic
are analyzed by direct injection of mercury vapor into the
mercury compounds.
instrument at a specified temperature on supported gold traps.
1.2 Both, inch-pound and SI (metric) units of measurement
are used throughout this standard.
4. Significance and Use
1.3 This standard does not purport to address all of the
4.1 This test method can be used to determine the total
safety concerns, if any, associated with its use. It is the
mercury concentration of a natural gas stream down to 0.001
responsibility of the user of this standard to establish appro-
µg/m . It can be used to assess compliance with environmental
priate safety and health practices and determine the applica-
regulations, predict possible damage to gas plant equipment,
bility of regulatory limitations prior to its use.
and monitor the efficiency of mercury removal beds.
2. Referenced Documents 4.2 The preferred sampling method for mercury collection
is on supported gold sorbent, which allows the element to be
2.1 ASTM Standards:
trapped and extracted from the interfering matrix of the gas.
D3684 Test Method for Total Mercury in Coal by the
Thermal desorption of mercury is performed by raising the
Oxygen BombCombustion/Atomic Absorption Method
temperature of the trap by means of a nichrome wire coiled
D5954 Test Method for Mercury Sampling and Measure-
around it.
ment in Natural Gas by Atomic Absorption Spectroscopy
4.3 Since AFS demonstrates lower detection limits ap-
2.2 ISO Standard:
proaching 0.1 pg, this test method avoids difficulties associated
ISO 6978 Determination of Mercury in Natural Gas
with prolonged sampling time. Saturation of the trap with
3. Summary of Test Method
interferantssuchashydrogensulfide(H S)isavoided.Average
sampling can range between 15 to 30 min, or less.
3.1 Mercury from the gaseous stream is absorbed and
preconcentrated onto a gold-coated silica sand trap. The
5. Apparatus and Materials
analyte is desorbed by raising the temperature of the trap, and
5.1 Sampling Equipment:
a flow of inert gas carries the mercury atoms into the cell
5.1.1 Sample probe, equipped with a ball valve of the Type
assembly of an atomic fluorescence spectrophotometer. The
316 SS, connected to the sampling point is highly recom-
cell is irradiated by a low pressure mercury vapor lamp at
mended.
5.1.2 Pressure regulation devices, such as a two-stage stain-
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
less steel pressure regulator, capable of reducing the pressure
Fuels and is the direct responsibility of Subcommittee D03.05 on Determination of
from 2000 to 30 psi.
Special Constituents of Gaseous Fuels.
5.1.3 On/off and micrometric-type valves capable of regu-
Current edition approved May 10, 2003. Published August 2003. Originally
approved in 1998. Previous edition approved in 1998 as D6350 – 98. DOI:
lating the natural gas sample flow rate in the range of 100 to
10.1520/D6350-98R03.
200 mL/min.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
5.1.4 Stainlesssteeltubingandcompression-typefittings,as
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
required.
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
5.1.5 Dry or wet flow meter or integrating anemometer to
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
measure properly the total volume of the gas sample collected.
4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D6350–98 (2003)
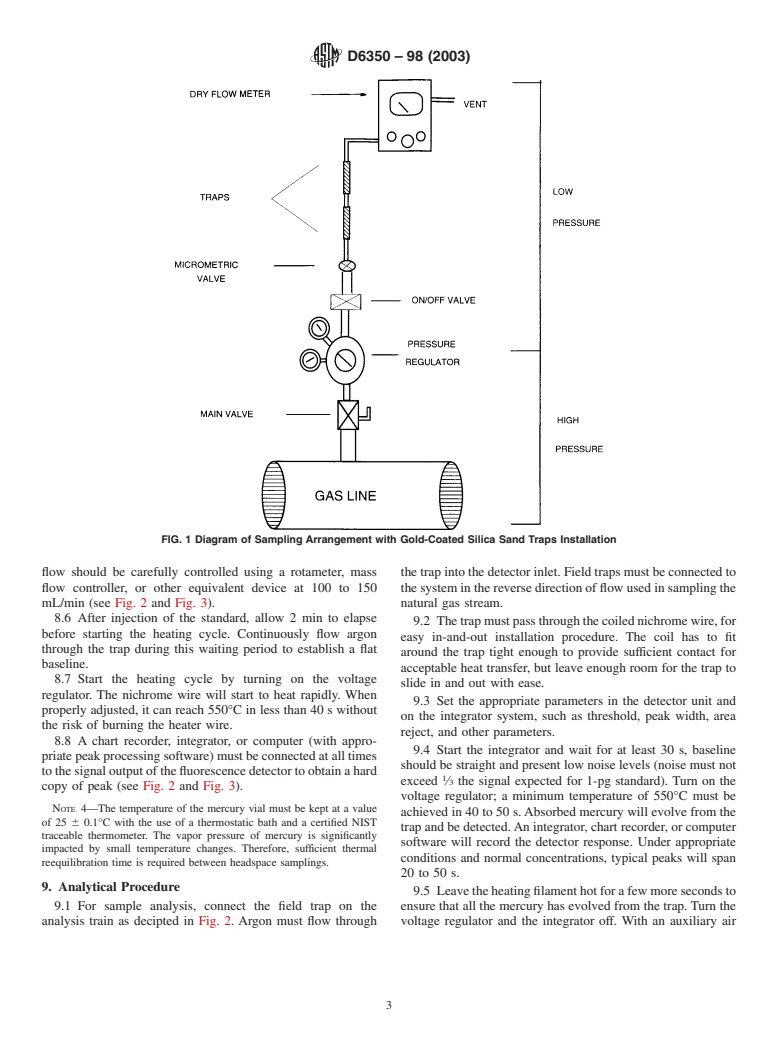
5.1.6 Gold-coated fused silica sand traps. 7.2 Sampling arrangements will always use a minimum of
two sampling gold tubes per location. The recommended
NOTE 1—For details on trap preparation refer to Test Method D5954,
sampling setup is shown schematically in Fig. 1.
the procedure of vapor deposition used in scanning electron microscopy
7.3 Assemble the parts without connecting the gold traps, as
(SEM) techniques, and, ISO 6978, 1993.
depicted in Fig. 1. Open the flow of gas from the main valve
5.2 Analytical Equipment:
and regulate the pressure down to 30 psi. Open the on/off valve
5.2.1 Atomic Fluorescence Spectrophotometer, equipped
and set an approximate flow of 150 mL/min with the micro-
with a quartz cell and a mercury lamp capable of irradiating at
metric valve adjustment. Check the flow with a dry or bubble
253.652-nm wavelength.
flow meter. Let the system purge for at least 30 min. Purging is
5.2.2 Chromatography Grade Teflont and Silicon Tubing,
necessary, especially if the pressure regulator, tubing, and
for connections between the thermal desorption system and the
valveswereusedat apreviouslocation.Thelongerthepurging
AFS. Length, ID, and OD are selected as appropriate.
period the better.
5.2.3 Nichrome Wire (22 gage) coiled (20 turns/inch)
7.4 When purging is completed, close the on/off valve and
around the traps for the thermal desorption of mercury.
connect both gold traps to the system. Use Tygon tubing, or
5.2.4 Variable Voltage Regulator, (rheostat) used in con-
something similar, to connect traps together.
junction with the nichrome wire for the rapid heating of the
7.5 Open the on/off valve again and record the time and the
traps.
exact flow through the traps. Check every 15 min that the flow
5.2.5 Temperature-Resistant Rubber Tubing,of ⁄4 in. (0.06
remains constant throughout the duration of sampling. Best
mm),connectingthetraptothetemperaturedesorptionsystem.
results are obtained with a 100- to 200-mL/min flow rate and
5.2.6 GC-Grade Septa, low bleed, made of silicone used in
an average sampling time of 15 to 30 min. Record both
the injection port and mercury-sealed vial.
readings.
5.2.7 Constant Temperature Bath, capable of regulating the
7.6 When sampling time has elapsed, close the on/off valve
temperature of a sealed vial of mercury to 25 6 0.1°C.
and disconnect the traps. Carefully cap and label them accord-
5.2.8 Various Stainless Steel “T” Fittings.
ingly (Tube 1 andTube 2).Accurately record the final time and
5.2.9 Gastight Syringes, fixed or variable volume, in the
flow data for later calculations.
range of 10 to 500 µL.
5.2.10 A Glass Vial, 100 mLfitted with a septum to perform
8. Calibration of the Instrument (Gaseous Standard)
as mercury container.
8.1 Calibration according to the following procedure is
5.2.11 Chart Recorder, or integrator to process a hard copy
recommended since it is easy to perform and results in
of the data acquired by the detector.
repeatability not exceeding a 10 % range between duplicate
NOTE 2—Commercially available permeation injection sources, based analyses. (see also ISO 6978).
on the principle of permeation tubes, can be used instead of gastight
8.2 Standards are prepared by injection of different volumes
syringes. Permeation devices can be used in lieu of gastight syringe-based
of the head space from a thermostated, sealed mercury vial.
sample introduction.Apermeation system can automatically introduce an
Injection of the aliquots, usually in the microlitre range, should
accurately known amount of mercury vapor onto a gold trap. This is
be made directly onto a mercury trapping tube, using a T-piece
particularly convenient for quantifying low pg amounts of mercury.
injection port and argon gas as carrier. See Fig. 2 for details.
6. Reagents
8.3 All surfaces coming in contact with the mercury vapor
should be passivated (except the analytical trap) before actual
6.1 Because of the error and contamination that may be
readings can be taken. Condition all tubing, instrument con-
introduced from impurities in the chemicals, the use of high
nections, as well as all syringes, by multiple injections of the
purity reagents is strongly recommended.
gaseous mercury vapor head space contained in the
6.1.1 Mercury Analytical Grade, triple distilled.
temperature-controlled mercury vial.
(Warning—Mercury vapor is harmful. Use proper ventilation
8.4 The concentration of a particular aliquot, taken with a
when handling.)
gastightsyringe,canbecalculatedbythefollowingequationof
6.2 Argon Gas, ultra high purity grade (UHP 99.999 %).
state of real gases:
NOTE 3—For the permeation i
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.