ASTM E1508-98(2008)
(Guide)Standard Guide for Quantitative Analysis by Energy-Dispersive Spectroscopy
Standard Guide for Quantitative Analysis by Energy-Dispersive Spectroscopy
SIGNIFICANCE AND USE
This guide covers procedures for quantifying the elemental composition of phases in a microstructure. It includes both methods that use standards as well as standardless methods, and it discusses the precision and accuracy that one can expect from the technique. The guide applies to EDS with a solid-state X-ray detector used on an SEM or EPMA.
EDS is a suitable technique for routine quantitative analysis of elements that are 1) heavier than or equal to sodium in atomic weight, 2) present in tenths of a percent or greater by weight, and 3) occupying a few cubic micrometres, or more, of the specimen. Elements of lower atomic number than sodium can be analyzed with either ultra-thin-window or windowless spectrometers, generally with less precision than is possible for heavier elements. Trace elements, defined as 1.0 %,2 can be analyzed but with lower precision compared with analyses of elements present in greater concentration.
SCOPE
1.1 This guide is intended to assist those using energy-dispersive spectroscopy (EDS) for quantitative analysis of materials with a scanning electron microscope (SEM) or electron probe microanalyzer (EPMA). It is not intended to substitute for a formal course of instruction, but rather to provide a guide to the capabilities and limitations of the technique and to its use. For a more detailed treatment of the subject, see Goldstein, et al. This guide does not cover EDS with a transmission electron microscope (TEM).
1.2 Units—The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1508–98 (Reapproved 2008)
Standard Guide for
Quantitative Analysis by Energy-Dispersive Spectroscopy
This standard is issued under the fixed designation E1508; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (ϵ) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2 Definitions of Terms Specific to This Standard:
3.2.1 accelerating voltage—the high voltage between the
1.1 This guide is intended to assist those using energy-
cathode and the anode in the electron gun of an electron beam
dispersive spectroscopy (EDS) for quantitative analysis of
instrument, such as an SEM or EPMA.
materials with a scanning electron microscope (SEM) or
3.2.2 beam current—the current of the electron beam mea-
electron probe microanalyzer (EPMA). It is not intended to
sured with a Faraday cup positioned near the specimen.
substitute for a formal course of instruction, but rather to
3.2.3 Bremsstrahlung—background X rays produced by
provide a guide to the capabilities and limitations of the
inelastic scattering (loss of energy) of the primary electron
technique and to its use. For a more detailed treatment of the
beam in the specimen. It covers a range of energies up to the
subject, see Goldstein, et al. This guide does not cover EDS
energy of the electron beam.
with a transmission electron microscope (TEM).
3.2.4 critical excitation voltage—the minimum voltage re-
1.2 Units—The values stated in SI units are to be regarded
quired to ionize an atom by ejecting an electron from a specific
as standard. No other units of measurement are included in this
electron shell.
standard.
3.2.5 dead time—the time during which the system will not
1.3 This standard does not purport to address all of the
process incoming X rays (real time less live time).
safety concerns, if any, associated with its use. It is the
3.2.6 k-ratio—the ratio of background-subtracted X-ray
responsibility of the user of this standard to establish appro-
intensity in the unknown specimen to that of the standard.
priate safety and health practices and determine the applica-
3.2.7 live time—the time that the system is available to
bility of regulatory limitations prior to use.
detect incoming X rays.
2. Referenced Documents 3.2.8 overvoltage—the ratio of accelerating voltage to the
critical excitation voltage for a particular X-ray line.
2.1 ASTM Standards:
3.2.9 shaping time—a measure of the time it takes the
E3 Guide for Preparation of Metallographic Specimens
amplifier to integrate the incoming charge; it depends on the
E7 Terminology Relating to Metallography
time constant of the circuitry.
E673 Terminology Relating to Surface Analysis
3.2.10 spectrum—the energy range of electromagnetic ra-
E691 Practice for Conducting an Interlaboratory Study to
diation produced by the method and, when graphically dis-
Determine the Precision of a Test Method
played, is the relationship of X-ray counts detected to X-ray
3. Terminology
energy.
3.1 Definitions—For definitions of terms used in this guide,
4. Summary of Practice
see Terminologies E7 and E673.
4.1 As high-energy electrons produced with an SEM or
EPMAinteract with the atoms within the top few micrometres
ThisguideisunderthejurisdictionofASTMCommitteeE04onMetallography
of a specimen surface, X rays are generated with an energy
and is the direct responsibility of Subcommittee E04.11 on X-Ray and Electron
Metallography. characteristic of the atom that produced them. The intensity of
Current edition approved June 1, 2008. Published September 2008. Originally
such X rays is proportional to the mass fraction of that element
approved in 1993. Last previous edition approved in 2003 as E1508 – 98(2003).
in the specimen. In energy-dispersive spectroscopy, X rays
DOI: 10.1520/E1508-98R08.
from the specimen are detected by a solid-state spectrometer
Goldstein,J.I.,Newbury,D.E.,Echlin,P.,Joy,D.C.,Romig,A.D.,Jr.,Lyman,
C. D., Fiori, C., and Lifshin, E., Scanning Electron Microscopy and X-ray
that converts them to electrical pulses proportional to the
Microanalysis, 3rd ed., Plenum Press, New York, 2003.
characteristic X-ray energies. If the X-ray intensity of each
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
element is compared to that of a standard of known composi-
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on tion and suitably corrected for the effects of other elements
the ASTM website.
present, then the mass fraction of each element can be
Withdrawn. The last approved version of this historical standard is referenced
calculated.
on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E1508–98 (2008)
5. Significance and Use suitable coating material. Heavy metals such as gold that are
often used for SEM imaging are less suitable because they
5.1 This guide covers procedures for quantifying the el-
heavily absorb X rays; if the coating is thick enough, X-ray
emental composition of phases in a microstructure. It includes
lines from those metals will be seen in the spectrum. If one is
both methods that use standards as well as standardless
analyzing carbon in the specimen, then aluminum makes a
methods, and it discusses the precision and accuracy that one
good coating. The coatings are usually applied in thicknesses
can expect from the technique. The guide applies to EDS with
of several tens of nanometres. Carbon that appears to be tan in
a solid-state X-ray detector used on an SEM or EPMA.
color on the specimen surface, or on a piece of filter paper in
5.2 EDS is a suitable technique for routine quantitative
theevaporator, isprobablythickenough. For themostaccurate
analysisofelementsthatare 1)heavierthanorequaltosodium
analysis,standardsandunknownsshouldbecoatedatthesame
in atomic weight, 2) present in tenths of a percent or greater by
time to assure equal coating thicknesses. Specimens mounted
weight, and 3) occupying a few cubic micrometres, or more, of
in a nonconducting medium must make electrical contact with
the specimen. Elements of lower atomic number than sodium
the microscope stage. This is often accomplished by painting a
can be analyzed with either ultra-thin-window or windowless
stripe of carbon or silver paint from the specimen to the
spectrometers,generallywithlessprecisionthanispossiblefor
specimen holder.
heavier elements. Trace elements, defined as <1.0 %, can be
analyzed but with lower precision compared with analyses of
8. Spectrum Collection
elements present in greater concentration.
8.1 Calibration—The analyzer shall be calibrated on two
6. Test Specimens
X-ray peaks or other methods implemented by the equipment
manufacturer in software to set the amplifier gain and offset.
6.1 Suitable specimens are those that are normally stable
Often aluminum and copper are used, and sometimes both the
under an electron beam and vacuum and are homogeneous
K and L lines of copper are used. The two elements need not
throughout the volume of X-ray production. If the specimen is
be in the same specimen. A spectrum from pure aluminum
inhomogeneous at the micrometre level, then a truly quantita-
could be collected followed by pure copper in the same
tiveanalysisisnotpossible,andabulktechniquesuchasX-ray
spectrum. Software is usually available to calibrate the EDS
fluorescence should be used.
system, and one should consult the system manual for the
6.2 The concentration of each element to be analyzed
details of operation. To ensure reproducible results, calibration
should equal or exceed about 0.1 wt %. Lower limits of
should be checked periodically.
detection are possible with longer counting times, but the
8.2 Operating Parameters:
precision of trace element analysis is poorer than when the
8.2.1 The accelerating voltage of the SEM must be chosen
element is present at the percent level.
to provide an adequate overvoltage to excite the X-ray lines of
7. Specimen Preparation
interest. An overvoltage that is too low will not sufficiently
7.1 Specimens for quantitative EDS analysis should be excite X rays; one that is too high yields low spatial resolution
prepared in accordance with standard metallographic or petro- and causes absorption as X rays escape from deep within the
graphic techniques. Guidelines are given in Methods E3. The specimen. An overvoltage of at least 1.5 times the critical
specimen must be flat in the region to be analyzed. This excitationpotentialofthehighestenergyX-raylineanalyzedis
requirement does not preclude scratches; however, any recommended. When analyzing hard and soft X rays in the
scratchesintheimmediatevicinityoftheanalyzedregionmust samespecimen,analysesattwovoltagesmaybenecessary.For
be insignificant with respect to the X-ray volume.The operator materials such as minerals and ceramics, which contain light
must also be aware of the possibility of spurious X rays from elements (that is, of low atomic number), 15 kV is usually a
parts of the chamber, polishing compound elements, or from goodcompromise.Formanymetalscontainingmediumatomic
number elements, 20 to 30 kV is a good choice. Heavy
adjacent phases or a combination thereof. Note that these
requirements for surface preparation preclude the quantitative elements (those of higher atomic number) may be analyzed
using L or M lines, and so higher voltages are not necessary.
analysis of casual samples, such as unpolished surfaces like
fracture surfaces. Although data can be generated on these The actual accelerating voltage of the electron beam does not
always correspond with the voltage selected on the instrument.
casual surfaces, the results would be of significantly lower
precision with unpredictable variations. ItcanbedeterminedbyexpandingtheverticalscaleoftheEDS
7.2 Unetched or lightly etched specimens are preferred. If spectrum and observing the energy above which continuum X
they are etched, the operator must make sure that the compo- rays do not occur.
sition in the region to be analyzed has not been altered and that 8.2.2 Almost all elements can be analyzed using character-
the region to be analyzed is flat. istic X-ray lines in the range of 0–10 keV. This range contains
7.3 Nonconducting specimens should be coated with a K lines of the first transition series (scandium–zinc (Sc-Zn)), L
conductive material to prevent charging. Lowering the accel- linesofthesecondtransitionseriesplusthelanthanides,andM
erating voltage may reduce or eliminate the effect of charging lines of the third transition series plus the actinides. Accord-
in some samples, but applying a conductive coating is still the ingly, most operators choose a 0–10 keV display at higher
most common method. Evaporated carbon is usually the most display resolution rather than a 0–20 keV display at lower
E1508–98 (2008)
resolution. Tables of X-ray energies can be found in various
2 5
texts, such as Goldstein, et al or Johnson and White.
8.2.3 X-ray spatial resolution degrades with overvoltage,
because as the electrons penetrate deeper into the specimen, X
rays are generated from a larger volume.An approximation of
the diameter of this tear-drop-shaped excitation volume, re-
ferred to as the X-ray range, can be obtained using the
following equation.
1.68 1.68
R 5 0.064~E 2 E !/r (1)
o c
where:
R = the range in µm,
E = the accelerating voltage in kV,
o
E = the critical excitation potential in keV, and
c
ρ = the density in g/cm .
More accurate interaction volumes can be computed by
Monte Carlo computer methods to generate random electron
trajectories, but Eq 1 provides a reasonable estimate for most
FIG. 1 Schematic Diagram of Electron Microscope System
purposes.
8.2.4 The beam can be placed in the spot mode to form a
probe to analyze the minimum volume, or it can be scanned
detector is directly proportional to the size of the solid angle
over a homogeneous region to lower the electron dose at any
defined as follows for a detector normal to the line of sight to
one point. Defocusing the beam or scanning it over an area of the specimen:
varying composition does not provide an average composition,
V5 A/r (2)
because the correction factors applied to the intensity ratio are
themselves a function of composition.
where:
8.2.5 Thecurrentintheelectronbeamdeterminesthefluxof Ω = solid angle in steradians,
A = active area of the detector crystal; for example, 30
X rays that are generated. It does not affect spatial resolution
mm , and
for X-ray analysis in the same way it detracts from electron
r = sample-to-detector distance, mm.
image resolution. Typically it is adjusted to keep the dead time
The larger the active area of the detector, the more counts
in the EDS system below 40 %. Dead times of 20 to 30 %
will be collected, but at the expense of spectral resolution.
producegoodspectra,whereasdeadtimesabove40 %canlead
Most detectors have a movable slide and can be brought closer
to spectra containing artifacts, such as those discussed in 8.3.1.
to the sample if a higher count rate at a given beam current is
Maximum throughput, that is, the most X rays/real time, is
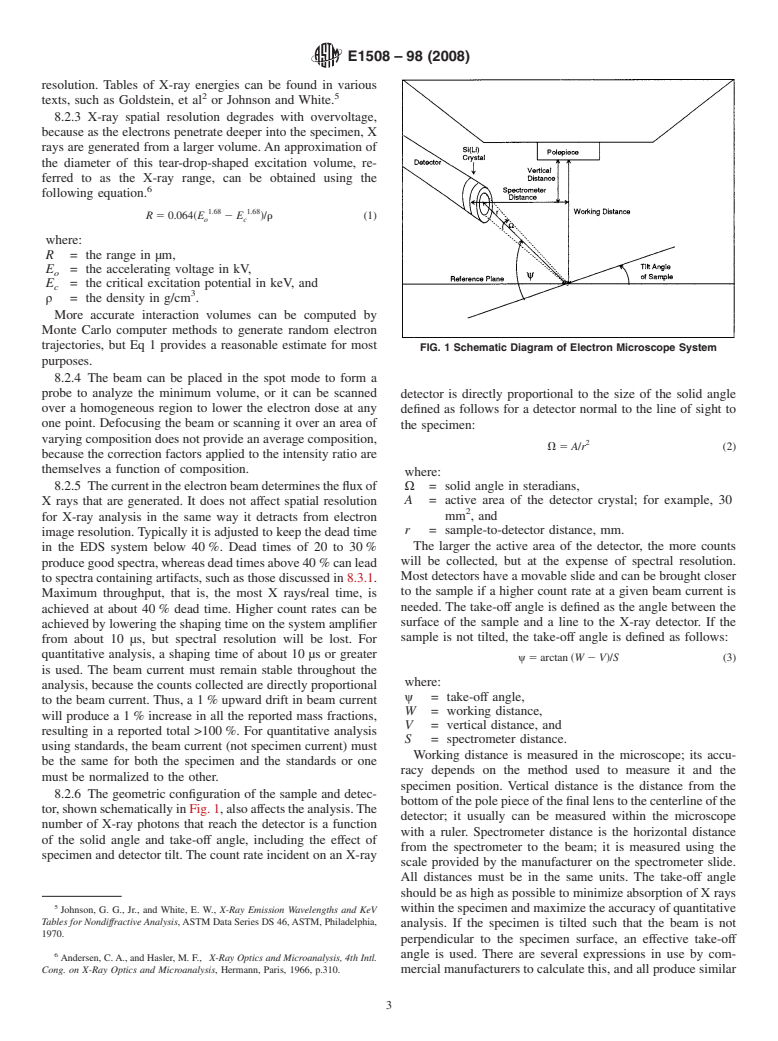
needed. The take-off angle is defined as the angle between the
achieved at about 40 % dead time. Higher count rates can be
surface of the sample and a line to the X-ray detector. If the
achieved by lowering the shaping time on the system amplifier
sample is not tilted, the take-off angle is defined as follows:
from about 10 µs, but spectral resolution will be lost. For
quantitative analysis, a shaping time of about 10 µs or greater
ψ 5 arctan ~W 2 V!/S (3)
is used. The beam current must remain stable throughout the
where:
analysis, because the counts collected are directly proportional
ψ = take-off angle,
to the beam current. Thus, a 1 % upward drift in beam current
W = working distance,
will produce a 1 % increase in all the reported mass fractions,
V = vertical distance, and
resulting in a reported total >100 %. For quantitative analysis
S = spectrometer distance.
using standards, the beam current (not specimen current) must
Working distance is measured in the microscope; its accu-
be the same for both the specimen and the standards or one
racy depends on the method used to measure it and the
must be normalized to the other.
specimen position. Vertical distance is the distance from the
8.2.6 The geometric configuration of the sample and detec-
bottomofthepolepieceofthefinallenstothecenterlineofthe
tor,shownschematicallyinFig.1,alsoaffe
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.