ASTM E537-98
(Test Method)Standard Test Method for Assessing the Thermal Stability of Chemicals By Methods of Thermal Analysis
Standard Test Method for Assessing the Thermal Stability of Chemicals By Methods of Thermal Analysis
SCOPE
1.1 This test method covers the ascertainment of the presence of enthalpic changes, using a minimum quantity of sample, normally in the milligram range, and approximates the temperature at which these enthalpic changes occur.
1.2 This test method utilizes techniques of differential thermal analysis (DTA) and differential scanning calorimetry (DSC); it may be performed on solids, liquids, or slurries.
1.3 This test method may be carried out in an inert or a reactive atmosphere with an absolute pressure range from 100 Pa through 7 MPa and over a temperature range from -150°C to above 1000°C.
1.4 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are given in Section 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: E 537 – 98
Standard Test Method for
Assessing The Thermal Stability Of Chemicals By Methods
Of Thermal Analysis
This standard is issued under the fixed designation E 537; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
Committee E-27 is currently engaged in developing methods to determine the hazard potential of
chemicals. An estimate of this potential may usually be obtained by the use of program CHETAH 7.0
to compute the maximum energy of reaction of the chemical or mixture of chemicals.
The expression “hazard potential” as used by this committee is defined as the degree of
susceptibility of material to ignition or release of energy under varying environmental conditions.
The primary purpose of this test method is to detect enthalpic changes and to approximate the
temperature of initiation of these events. Thermal analysis techniques including differential thermal
analysis (DTA) and differential scanning calorimetry (DSC) offer the advantage of using very small
samples on the order of a few milligrams.
1. Scope E 473 Terminology Relating to Thermal Analysis
E 967 Practice for Temperature Calibration of Differential
1.1 This test method covers the ascertainment of the pres-
Scanning Calorimeters and Differential Thermal Analyz-
ence of enthalpic changes, using a minimum quantity of
ers
sample, normally in the milligram range, and approximates the
E 1445 Terminology Relating to Hazardous Potential of
temperature at which these enthalpic changes occur.
Chemicals
1.2 This test method utilizes techniques of differential
E 1860 Test Method for Elapsed Time Calibration of Ther-
thermal analysis (DTA) and differential scanning calorimetry
mal Analyzers
(DSC); it may be performed on solids, liquids, or slurries.
1.3 This test method may be carried out in an inert or a
3. Terminology
reactive atmosphere with an absolute pressure range from 100
3.1 Definitions:
Pa through 7 MPa and over a temperature range from −150°C
3.1.1 Specific technical terms used in this standard are
to above 1000°C.
defined in Terminologies E 473 and E 1445.
1.4 The values stated in SI units are to be regarded as the
3.2 Definitions of Terms Specific to This Standard:
standard.
3.2.1 DTA (DSC) curve—a record of a thermal analysis
1.5 This standard may involve hazardous materials, opera-
where the temperature difference (DT) or the change in heat
tions, and equipment. This standard does not purport to
flow. (Dq) is plotted on the ordinate and temperature or time is
address all of the safety concerns associated with its use. It is
plotted on the abscissa (see Figs. 1 and 2 and Terminology
the responsibility of the user of this standard to establish
E 473).
appropriate safety and health practices and determine the
3.2.2 peak—that portion of a heating curve which is attrib-
applicability of regulatory limitations prior to use. Specific
utable to the occurrence of a single process. It is normally
safety precautions are given in Section 8.
characterized by a deviation from the established baseline, a
2. Referenced Documents maximum deflection, and a reestablishment of a baseline not
necessarily identical to that before the peak (see Fig. 1).
2.1 ASTM Standards:
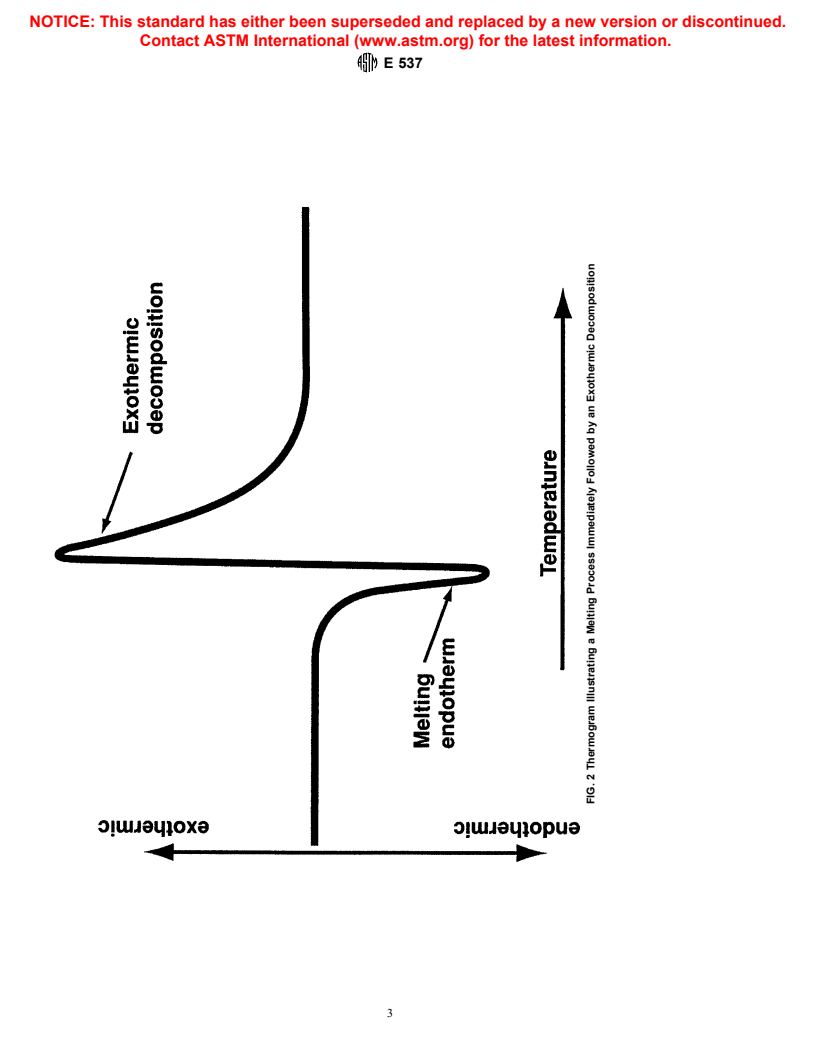
NOTE 1—There will be instances when upon scanning in temperature
an endotherm will be observed that is immediately followed by or is in
This test method is under the jurisdiction of ASTM Committee E-27 on Hazard conjunction with an exotherm as shown in Fig. 2. These types of
Potential of Chemicalsand is the direct responsibility of Subcommittee E27.02on
competing reactions make it difficult and at times impossible to locate the
Thermal Stability.
true peak and onset temperatures.
Current edition approved July 10, 1998. Published December 1998. Originally
3.2.3 peak temperature—the temperature corresponding to
published as E 537 – 76. Last previous edition E 537 – 86 (1992).
A complete assessment of the hazard potential of chemicals must take into
account a number of realistic factors not considered in this test method or the
CHETAH program. Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
E 537
FIG. 1 Typical DTA—DSC Curve with Exotherm
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
E 537
FIG. 2 Thermogram Illustrating a Melting Process Immediately Followed by an Exothermic Decomposition
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
E 537
the maximum deflection of the DTA or DSC curve. position is often observed.
3.2.4 onset temperature—the temperature at which a deflec- 5.4 For some substances the rate of enthalpy change during
tion from the established baseline is first observed. an exothermic reaction may be small at normal atmospheric
3.2.5 extrapolated onset temperature—empirically, the tem- pressure, making an assessment of the temperature of instabil-
perature found by extrapolating the baseline (prior to the peak) ity difficult. Generally a repeated analysis at an elevated
and the leading side of the peak to their intersection (see Fig. pressure will improve the assessment by increasing the rate of
1). change of enthalpy.
3.2.6 reaction—any transformation of material accompa-
NOTE 2—The choice of pressure may sometimes be estimated by the
nied by a change of enthalpy which may be endothermic or
pressure of the application to which the material is exposed.
exothermic.
5.5 Although certain types of thermal analysis instrumenta-
3.2.7 thermal stability—the absence of a reaction (for the
tion offer the additional advantage of measuring the magnitude
purposes of this test method only, see 3.2.6).
of the change in enthalpy, such measurements are beyond the
scope of this test method. The three significant criteria of this
4. Summary of Test Method
test method are: the detection of a change of enthalpy; the
4.1 In DTA, thermocouples for both the sample and refer-
approximate temperature at which the event occurs; and the
ence material are connected in series-opposition so as to
observance of effects due to the cell atmosphere and pressure.
measure a temperature difference (DT). An additional thermo-
couple is provided to measure the absolute temperature (T)of
6. Limitations
the sample or reference.
6.1 A host of environmental factors affect the existence,
4.2 In DSC, a measurement is made of the heat flow (Dq)
magnitude, and temperature of an exothermic reaction. Some,
associated with the observed change of enthalpy. Provisions
including heating rate, instrument sensitivity, degree of con-
are made to measure the absolute temperature ( T)ofthe
finement, and atmosphere reactivity will affect the detectability
sample or reference or the average temperature of both.
of an exothermic reaction using this procedure. Therefore, it is
4.3 A sample of the material to be examined and of a
imperative that the qualitative results obtained from the appli-
thermally inert reference material are placed in separate
cation of this test method be viewed only as an indication of the
holders.
thermal stability of a chemical.
4.4 The sample and reference materials are simultaneously
heated at a controlled rate of 2 to 30°C/min under an
7. Apparatus
equilibrated atmosphere. A record of DT or Dq on the ordinate
7.1 The equipment used in this test method shall be capable
is made as a function of temperature (T) on the abscissa.
of displaying changes of enthalpy as a function of temperature
4.5 When the sample undergoes a transition involving a
(T), and shall have the capability of subjecting the sample cell
change of enthalpy, that change is indicated by a departure
to different atmospheres of equilibrated pressures.
from the initially established baseline of the temperature
7.2 The differential thermal analytical instrument (DTA or
record.
DSC) may be purchased or custom built to various degrees of
refinement and sophistication. The basic components of an
5. Significance and Use
apparatus satisfactory for this test method include:
5.1 This test method is useful in detecting potentially
7.2.1 A test chamber composed of:
hazardous reactions including those from volatile chemicals
7.2.1.1 Furnace(s), to provide uniform controlled heating
and in estimating the temperatures at which these reactions
(cooling) of a specimen and reference to a constant temperature
occur. This test method is recommended as an early test for
or at a constant rate within the applicable temperature range of
detecting the thermal hazards of an uncharacterized chemical
this method,
substance or mixture (see Section 8).
7.2.1.2 Temperature sensor, to provide an indication of the
5.2 The magnitude of the change of enthalpy may not
specimen/furnace temperature to 60.5 K,
necessarily denote the relative hazard in a particular applica-
7.2.1.3 Differential sensor, to detect a temperature or heat
tion. For example, certain exothermic reactions are often
flow difference between the specimen and reference equivalent
accompanied by gas evolution which increases the potential
to 0.2 mW,
hazard. Alternatively, the extent of energy release for certain
7.2.1.4 Means of sustaining a test chamber environment of
exothermic reactions may differ widely with the extent of
inert (for example, nitrogen, helium or argon) or reactive (for
confinement of volatile products. Thus, the presence of an
example, air) gas at a purge rate of 50 6 5 mL/min,
exotherm or of an endotherm and its approximate temperature
are the most significant criteria in this test method (see Section
NOTE 3—Typically, at least 99 % pure nitrogen, argon or helium is
employed when oxidation in air is a concern. Unless effects of moisture
3 and Fig. 1).
are to be studied, use of dry purge gas is recommended and is essential for
5.3 When volatile substances are being studied, it is impor-
operation at subambient temperatures.
tant to perform this test with a confining pressurized atmo-
sphere so that changes of enthalpy which can occur above 7.2.1.5 Temperature controller, capable of executing a spe-
normal boiling or sublimation points may be detected. As an cific temperature program by operating the furnace(s) betwe
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.