ASTM E537-02
(Test Method)Standard Test Method for The Thermal Stability Of Chemicals By Differential Scanning Calorimetry
Standard Test Method for The Thermal Stability Of Chemicals By Differential Scanning Calorimetry
SIGNIFICANCE AND USE
This test method is useful in detecting potentially hazardous reactions including those from volatile chemicals and in estimating the temperatures at which these reactions occur and their enthalpies (heats). This test method is recommended as an early test for detecting the thermal hazards of an uncharacterized chemical substance or mixture (see Section 8).
The magnitude of the change of enthalpy may not necessarily denote the relative hazard in a particular application. For example, certain exothermic reactions are often accompanied by gas evolution that increases the potential hazard. Alternatively, the extent of energy release for certain exothermic reactions may differ widely with the extent of confinement of volatile products. Thus, the presence of an exotherm and its approximate temperature are the most significant criteria in this test method (see Section 3 and Fig. 1).
When volatile substances are being studied, it is important to perform this test with a confining pressurized atmosphere so that changes of enthalpy that can occur above normal boiling or sublimation points may be detected. As an example, an absolute pressure of 1.14 MPa (150 psig) will generally elevate the boiling point of a volatile organic substance 100 °C. Under these conditions exothermic decomposition is often observed.
For some substances the rate of enthalpy change during an exothermic reaction may be small at normal atmospheric pressure, making an assessment of the temperature of instability difficult. Generally a repeated analysis at an elevated pressure will improve the assessment by increasing the rate of change of enthalpy.
Note 2—The choice of pressure may sometimes be estimated by the pressure of the application to which the material is exposed.
The four significant criteria of this test method are: the detection of a change of enthalpy; the approximate temperature at which the event occurs; the estimation of its enthalpy and the observance of effects due to th...
SCOPE
1.1 This test method covers the ascertainment of the presence of enthalpic changes in a test specimen, using minimum quantities of material, approximates the temperature at which these enthalpic changes occur and determines their enthalpies (heats) using differential scanning calorimetry or pressure differential scanning calorimetry.
1.2 This test method may be performed on solids, liquids, or slurries.
1.3 This test method may be performed in an inert or a reactive atmsophere with an absolute pressure range from 100 Pa through 7 MPa and over a temperature range from 300 to 800 K (27 to 527°C ).
1.4 SI values are the standard.
1.5 There is no ISO standard equivalent to this test method.
1.6 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are given in Section 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E 537–02
Standard Test Method for
The Thermal Stability Of Chemicals By Differential Scanning
1
Calorimetry
This standard is issued under the fixed designation E 537; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
INTRODUCTION
Committee E-27 is currently engaged in developing methods to determine the hazard potential of
chemicals.An estimate of this potential may usually be obtained by the use of program CHETAH 7.0
2
to compute the maximum energy of reaction of the chemical or mixture of chemicals.
The expression “hazard potential” as used by this committee is defined as the degree of
susceptibility of material to ignition or release of energy under varying environmental conditions.
The primary purpose of this test method is to detect enthalpic changes and to approximate the
temperatureofinitiationandenthalpies(heats)oftheseevents.Differentialscanningcalorimetryoffers
the advantage of using very small specimens on the order of a few milligrams.
1. Scope 2. Referenced Documents
1.1 This test method covers the ascertainment of the pres- 2.1 ASTM Standards:
3
ence of enthalpic changes in a test specimen, using minimum E 473 Terminology Relating to Thermal Analysis
quantities of material, approximates the temperature at which E 691 Practice for Conducting an Interlaboratory Study to
3
these enthalpic changes occur and determines their enthalpies Determine the Precision of a Test Method
(heats) using differential scanning calorimetry or pressure E 967 Practice for Temperature Calibration of Differential
differential scanning calorimetry. Scanning Calorimeters and Differential Thermal Analyz-
3
1.2 Thistestmethodmaybeperformedonsolids,liquids,or ers
slurries. E 968 Practice for Heat Flow Calibration of Differential
3
1.3 This test method may be performed in an inert or a Scanning Calorimeters
reactive atmsophere with an absolute pressure range from 100 E 1445 Terminology Relating to Hazardous Potential of
3
Pa through 7 MPa and over a temperature range from 300 to Chemicals
800 K (27 to 527°C ). E 1860 Test Method for Elapsed Time Calibration of Ther-
3
1.4 SI values are the standard. mal Analyzers
1.5 There is no ISO standard equivalent to this test method.
3. Terminology
1.6 This standard may involve hazardous materials, opera-
tions, and equipment. This standard does not purport to 3.1 Definitions:
address all of the safety concerns associated with its use. It is 3.1.1 Specific technical terms used in this standard are
defined in Terminologies E 473 and E 1445.
the responsibility of the user of this standard to establish
appropriate safety and health practices and determine the 3.2 Definitions of Terms Specific to This Standard:
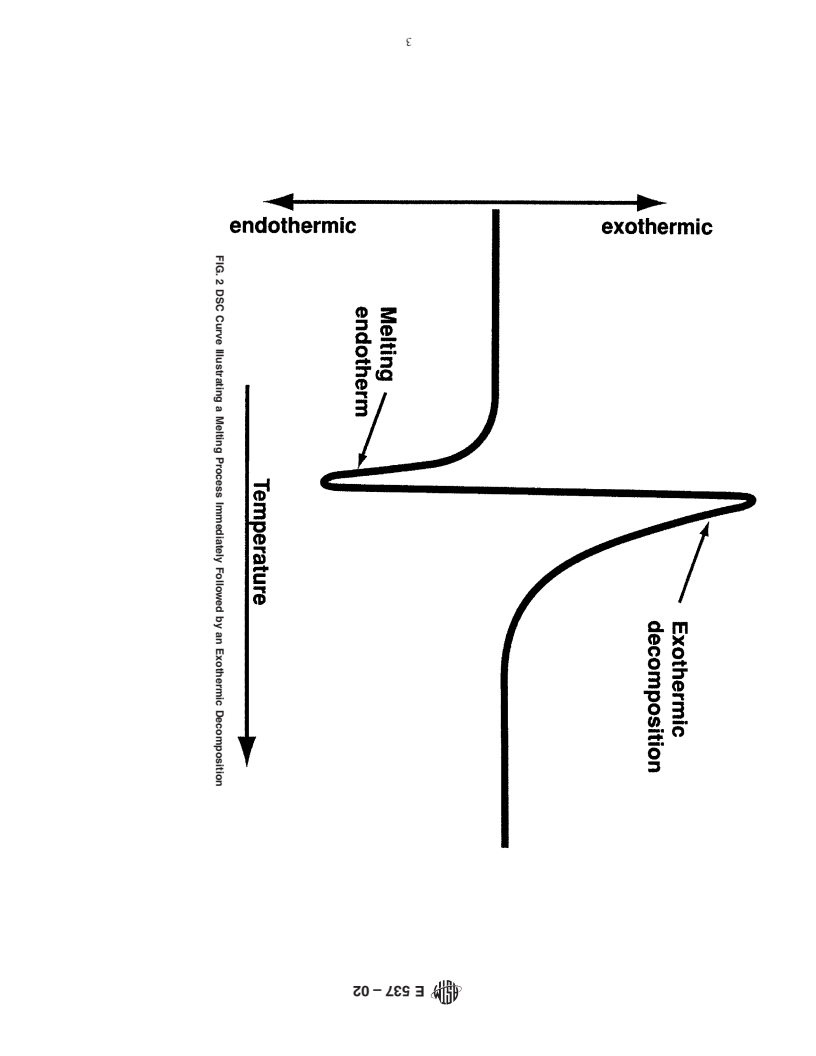
3.2.1 DSC curve—a record of a differential scanning calo-
applicability of regulatory limitations prior to use. Specific
safety precautions are given in Section 8. rimeter where the change in heat flow (Dq) is plotted on the
ordinate and temperature or time is plotted on the abscissa (see
Figs. 1 and 2 and Terminology E 473).
1
This test method is under the jurisdiction ofASTM Committee E27 on Hazard
3.2.2 peak—that portion of a thermal curve that is attribut-
Potential of Chemicals and is the direct responsibility of Subcommittee E27.02 on
Thermal Stability and Condensed Phases. able to the occurrence of a single process. It is normally
Current edition approved April 10, 2002. Published August 2002. Originally
characterized by a deviation from the established baseline, a
published as E 537 – 76. Last previous edition E 537 – 98.
2
A complete assessment of the hazard potential of chemicals must take into
account a number of realistic factors not considered in this test method or the
3
CHETAH program. Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
E537–02
2
FIG. 1 Typical DSC Curve with Exotherm
---------------------- Page: 2 ----------------------
E537–02
3
FIG. 2 DSC Curve Illustrating a Melting Process Immediately Followed by an Exothermic Decomposition
---------------------- Page: 3 ----------------------
E537–02
maximum deflection, and a reestablishment of a baseline not boiling or sublimation points may be detected.As an example,
necessarily identical to that before the peak (see Fig. 1). an absolute pressure of 1.14 MPa (150 psig) will generally
elevate the boiling point of a volatile organic substance 100°C.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.