ASTM D1070-03(2010)
(Test Method)Standard Test Methods for Relative Density of Gaseous Fuels
Standard Test Methods for Relative Density of Gaseous Fuels
SIGNIFICANCE AND USE
These test methods provide accurate and reliable methods to measure the relative density of gaseous fuels on an intermittent or continuous basis. These measurements are frequently used for regulatory or contract compliance custody transfer and process control.
SCOPE
1.1 These test methods cover the determination of relative density of gaseous fuels, including liquefied petroleum gases, in the gaseous state at normal temperatures and pressures. The test methods specified are sufficiently varied in nature so that one or more may be used for laboratory, control, reference, gas measurement, or in fact, for any purpose in which it is desired to know the relative density of gas or gases as compared to the density of dry air at the same temperature and pressure.
1.2 The procedures appear in the following sections:
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D1070 − 03 (Reapproved 2010)
Standard Test Methods for
Relative Density of Gaseous Fuels
This standard is issued under the fixed designation D1070; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 These test methods cover the determination of relative 3.1 Definitions:
density of gaseous fuels, including liquefied petroleum gases,
3.1.1 density—mass per unit of volume of the fuel gas or air
in the gaseous state at normal temperatures and pressures. The
being considered.
test methods specified are sufficiently varied in nature so that
3.1.2 gaseous fuel—material to be tested, as sampled, with-
one or more may be used for laboratory, control, reference, gas
out change of composition by drying or otherwise.
measurement, or in fact, for any purpose in which it is desired
3.1.3 relative density—ratio of the density of the gaseous
to know the relative density of gas or gases as compared to the
fuel, under the observed conditions of temperature and
density of dry air at the same temperature and pressure.
pressure, to the density of dried air, of normal carbon dioxide
1.2 The procedures appear in the following sections:
content, at the same temperature and pressure.
Section
3.1.3.1 Discussion—In these test methods the term “relative
Method A, Ac-Me Gravity Balance 7–9
Method B, Ranarex Recording and Indicating Gravitometer 10-11 density” has replaced the term “specific gravity.” The term,
Method C, UGC Gravitometer 12–14
specific gravity, as used in a previous edition of these test
NOTE 1—The test methods and apparatus described herein are repre- methods, was used incorrectly.
sentative of methods and apparatus used broadly in industry. Manufactur-
3.1.4 relative humidity—ratio of actual pressure of existing
er’s instructions for specific models should be consulted for further details
water vapor to maximum possible pressure of water vapor in
and as supplements to the information presented here. In addition to
instrumentation described below additional equally accurate and satisfac-
the atmosphere at the same temperature, expressed as a
tory instruments may be available.
percentage.
1.3 The values stated in inch-pound units are to be regarded
as standard. The values given in parentheses are mathematical 4. Summary of Test Methods
conversions to SI units that are provided for information only
4.1 Displacement Balances—This test method is based on
and are not considered standard.
the balancing of the weight of a fixed volume of gas at
1.4 This standard does not purport to address all of the
atmospheric pressure against the weight of dry air across the
safety concerns, if any, associated with its use. It is the
center of gravity of a balance beam. The amount of this
responsibility of the user of this standard to establish appro-
“deflection,” subject to correction, for humidity, high CO
priate safety and health practices and determine the applica-
content or other factor measures the relative density. Instru-
bility of regulatory limitations prior to use.
ments of this class may be either visual or chart recording.
4.2 Kinetic Energy—This test method measures the ratio of
2. Referenced Documents
the change in kinetic energy between an impeller and an
2.1 ASTM Standards:
impulse wheel operating in gas and a second impeller and
D5503 Practice for Natural Gas Sample-Handling and Con-
impulse wheel operating in a reference gas (generally air). The
ditioning Systems for Pipeline Instrumentation
relative torque of the impulse wheels is measured and provides
a value for relative density since the relative torque is propor-
tional to the gas and air densities.
These test methods are under the jurisdiction of ASTM Committee D03 on
Gaseous Fuels and is the direct responsibility of Subcommittee D03.03 on
Determination of Heating Value and Relative Density of Gaseous Fuels. 5. Significance and Use
CurrenteditionapprovedMay1,2010.PublishedJuly2010.Originallyapproved
5.1 These test methods provide accurate and reliable meth-
in 1952. Last previous edition approved in 2003 as D1070 – 03 . DOI: 10.1520/
D1070-03R10.
ods to measure the relative density of gaseous fuels on an
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
intermittent or continuous basis. These measurements are
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
frequently used for regulatory or contract compliance custody
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. transfer and process control.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D1070 − 03 (2010)
6. Sampling 8.1.2.3 Observe the scale from such a position that the
reflection of your eye in the look glass is centered on the
6.1 The sample shall be representative of the gas to be
hairline. Admit air through first valve and air dryer until the
measured and shall be taken from its source without change in
beam begins to fall. Then pinch down the flow of air through
form or composition. Sampling of natural gases should be in
first valve so that the air can be cut off at exactly the right
accordance with Practice D5503.
instant to keep the beam in the balanced position. Observe the
scale noting how far the zero swings above and below the
METHOD A—Ac–Me GRAVITY BALANCE
hairline. The beam is balanced when the zero of the scale is
(Four-Spring Type)
swinging an equal amount above and below the hairline.
8.1.2.4 When balance is obtained, lock instrument and read
7. Apparatus
and record the air vacuum shown on the manometer. Record
7.1 Ac–Me Gravity Balance (Four-Spring Type), pressure-
the temperature within the balance.
tight cylindrical container mounted on a base board. Inside the
8.1.3 Gas Reading:
container is a balance beam with a sealed float at the back and
8.1.3.1 Close the valve on the air dryer and close Valve 1.
graduated scale at the front. The beam is suspended at the
Then open Valve 2 and pull a vacuum of about 650 mm on the
center by thin flat springs. A window for viewing the scale is
balance.
provided at the front of the container. The balance beam may
8.1.3.2 Open gas supply valve and admit gas to the balance
be locked by a cam mechanism when the instrument is not in
until the pressure reads about 650 mm. (Do not exceed
use. Valves for introducing gas and air samples are provided.
manometer maximum reading or the balance may be dam-
7.2 Carrying Case, for transportation or storage.
aged.)
8.1.3.3 Repeat 8.1.3.1 and 8.1.3.2 three times. The third
7.3 Air Dryer, to dehydrate air samples (silica gel).
time will leave only about 0.05 % air in the balance. If the
7.4 Tripod, to support the balance firmly.
balance is purged by flowing gas through it, the purging should
7.5 Pressure-Vacuum Pump, to transfer samples and adjust be continued until two successive readings (8.1.3.4) check.
pressure in the balance.
8.1.3.4 Unlock the instrument and release gas pressure
through Valve 2 until balanced position of beam is reached.
7.6 Mercury Manometer, 760 mm, to measure pressure in
Follow the same method as described for the air reading in
the balance.
8.1.2.4. When balance is obtained, lock the instrument and
7.7 Aneroid Barometer,temperaturecompensatedtoconvert
record the gas pressure shown on the manometer. Record the
balance pressure readings to absolute pressures. (Absolute
temperature in the balance.
pressure not corrected to sea level.)
NOTE 2—When the gas supply is under a vacuum or has a high content
7.8 Rubber Hose, 6.35-mm ( ⁄4-in.) inside diameter, four
of hydrocarbons heavier than ethane, keep the gas pressure within the
lengths with brass swivel connections to join the balance to its balance below that in the source line or container to avoid condensation in
the balance. If necessary, readjust instrument to balance on gas at a
operating accessories.
vacuum about 20 mm higher than that in the sampling source.
7.9 Sampling Hose, 6.35 mm ( ⁄4 in.) with swivel connec-
8.1.4 Air Check Reading:
tions and two male 6.35-mm ( ⁄4-in.) pipe adapters.
8.1.4.1 Closegassupplyvalves.Opensecondvalveandpull
7.10 Additional Apparatus—Refer to the manufacturer’s
a vacuum of about 650 mm.
literature for further information on sizes, assembly, and other
8.1.4.2 Admit air through the air dryer to the balance until
details applicable to specific models.
atmospheric pressure is reached. Close first valve.
8.1.4.3 Repeat 8.1.4.1 and 8.1.4.2 at least three times or
8. Procedure
until two successive readings (8.1.4.4) will check.
8.1.4.4 Open second valve and pull a vacuum of about 650
8.1 Assemble and set up the balance in accordance with the
nm, then close second valve. Unlock instrument; admit air
manufacturer’s instructions, making certain that it is firmly
through first valve to bring the beam to the balanced position
supported, level, and is not disturbed during the entire test.
as when taking the first air reading.
Take and record the following four readings:
8.1.4.5 When balance is obtained, lock instrument; read and
8.1.1 Average Barometric Reading—Read the aneroid ba-
record the air vacuum shown on the manometer. Record the
rometer at the beginning and end of each test, and record the
temperature in the balance. This reading must check with the
average of these two readings.
first air reading if the two temperatures in the balance are the
8.1.2 Air Reading:
same. When test is complete close all valves on the balance.
8.1.2.1 Admit air through first valve and air dryer until
Close the cock on the air dryer to prevent moistening of silica
atmospheric pressure is reached. Record temperature in the
gel.
balance. Close first valve.
8.1.2.2 Open second valve and pull a vacuum of about 650
9. Calculation
mm, then close second valve. Unlock the balance beam by
turning locking level counterclockwise. The beam will then be 9.1 Whenananeroidbarometerisusedinthefield,itshould
in an unbalanced position with the zero above the hairline be checked periodically with a mercury barometer.The barom-
indicator. eter should be handled very carefully and be well packed for
D1070 − 03 (2010)
transportation. If barometer reading is in inches and fractions,
multiply reading by 25.4 to convert to millimetres. To convert
to absolute pressure, add barometric pressure in millimetres to
both air and gas pressure readings. (If air or gas reading is on
vacuum, subtract it from barometric pressure.) Divide the
absolute pressure for air by the absolute pressure for gas to
obtain the relative density of the gases shown in the following
example:
Manometer Barometer Absolute
Reading Reading Pressure
Barometer reading:
753 mm
Air reading −127 + 753 = 626
Gas reading 204 + 753 = 957
Air check reading −127
absolute air pressure
FIG. 1 Examples of Portable and Recording Ranarex Gravitom-
Relative density 5 (1)
absolute gas pressure 5 626/957 5 0.654 eters
9.2 When there is a difference between the temperature for
the air reading and the temperature for the gas reading, these
the impeller in the upper chamber draws in a continuous flow
temperature readings should be converted to absolute
of outside air and rotates it at the same speed as the gas but in
temperature, by adding 460, and used in calculations as shown
opposite direction.As the rotating air impinges on the impulse
by the following example:
wheel vanes, it too undergoes a change in kinetic energy that
Absolute Absolute
creates on the upper impulse wheel, a torque proportional to
Manometer Pressure, Temperature Temperature,
the density of the air.
Reading P °F T
Barometer reading: 10.1.3 The impulse wheel torques are transmitted through
745 mm
pivot shafts to the external lever arms, connecting link, and
Air reading −95 650 66 526
indicator, which move as a system to an angular position at
Gas reading 197 942 68 528
Air check reading −90 655 70 530
which the torques balance each other. The linkage system
serves as a mechanical computer dividing one torque by the
Relative density 5 ~P air/P gas! 3 ~T gas/T air! (2)
other. At each angular position of the linkage, there is a
corresponding value for the ratio. However, since the torques
5~650/942! 3 ~528/526! 5 0.693 ~first air reading!
are proportional to the density of the medium in each chamber,
Relative density 5 655/942 3 528/530 (3)
~ ! ~ !
the ratio may be expressed as follows:
50.693 air check reading
~ !
density of lower chamber/density of upper chamber (4)
METHOD B—RANAREX PORTABLE AND
STATIONARY GRAVITOMETERS
10. Apparatus
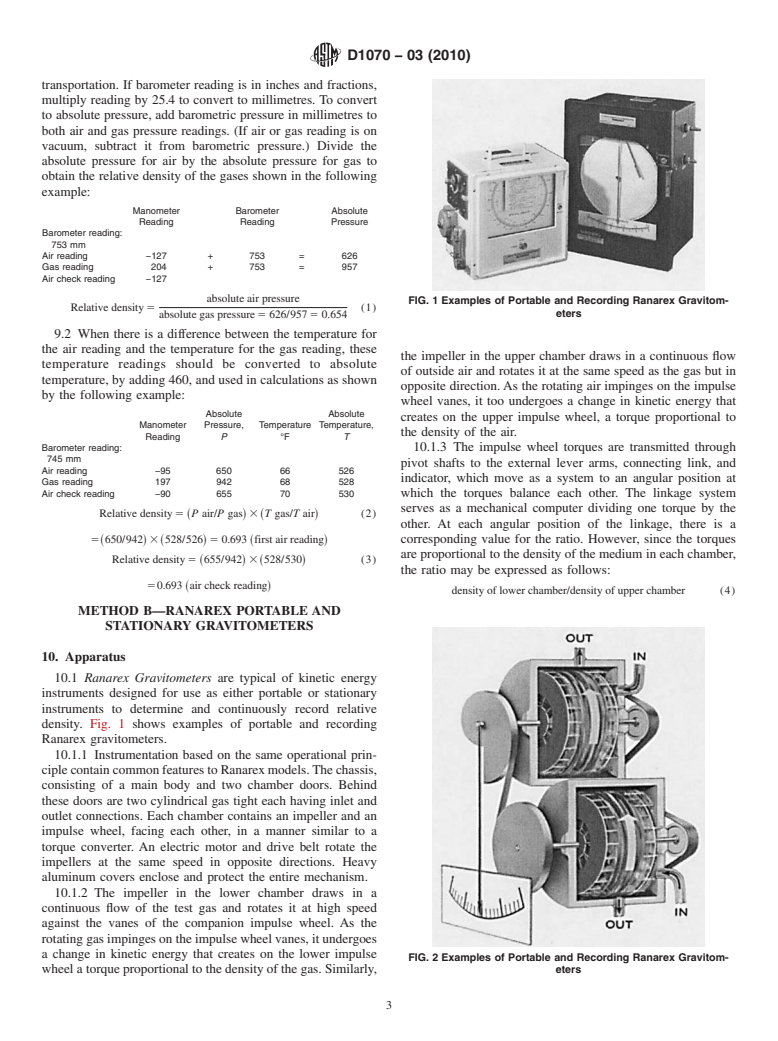
10.1 Ranarex Gravitometers are typical of kinetic energy
instruments designed for use as either portable or stationary
instruments to determine and continuously record relative
density. Fig. 1 shows examples of portable and recording
Ranarex gravitometers.
10.1.1 Instrumentation based on the same operational prin-
ciplecontaincommonfeaturestoRanarexmodels.Thechassis,
consisting of a main body and two chamber doors. Behind
these doors are two cylindrical gas tight each having inlet and
outlet connections. Each chamber contains an impeller and an
impulse wheel, facing each other, in a manner similar to a
torque converter. An electric motor and drive belt rotate the
impellers at the same speed in opposite directions. Heavy
aluminum covers enclose and protect the entire mechanism.
10.1.2 The impeller in the lower chamber draws in a
continuous flow of the test gas and rotates it at high speed
against the vanes of the companion impulse wheel. As the
rotating gas impinges on the impulse wheel vanes, it undergoes
a change in kinetic energy that creates on the lower impulse
FIG. 2 Examples of Portable and Recording Ranarex Gravitom-
wheel a torque proportional to the density of the gas. Similarly, eters
D1070 − 03 (2010)
10.1.4 When the unknown gas is admitted to the lower balance, and at equal distance from the pivot point, are two
chamber and air is admitted to the upper chamber, the ratio identical floats. One float is specific and contains a gas sample
becomes as follows: with a known relative density. The other float is the sample
float through which is passed a sample of the gas that is being
density of gas/density of air 5 relative density (5)
measured. The pressure in the two floats is kept equal by a
10.1.5 The relation between the value of this fraction and
pressure loaded regulator. This equalization of pressure pre-
angular position of the linkage an
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.