ASTM E840-95
(Practice)Standard Practice for Using Flame Photometric Detectors in Gas Chromatography
Standard Practice for Using Flame Photometric Detectors in Gas Chromatography
SCOPE
1.1 This practice is intended as a guide for the use of a flame photometric detector (FPD) as the detection component of a gas chromatographic system.
1.2 This practice is directly applicable to an FPD that employs a hydrogen-air flame burner, an optical filter for selective spectral viewing of light emitted by the flame, and a photomultiplier tube for measuring the intensity of light emitted.
1.3 This practice describes the most frequent use of the FPD which is as an element-specific detector for compounds containing sulfur (S) or phosphorus (P) atoms. However, nomenclature described in this practice are also applicable to uses of the FPD other than sulfur or phosphorus specific detection.
1.4 This practice is intended to describe the operation and performance of the FPD itself independently of the chromatographic column. However, the performance of the detector is described in terms which the analyst can use to predict overall system performance when the detector is coupled to the column and other chromatographic system components.
1.5 For general gas chromatographic procedures, Practice E260 should be followed except where specific changes are recommended herein for use of an FPD.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific safety information, see Section 4, Hazards.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: E 840 – 95
Standard Practice for
Using Flame Photometric Detectors in Gas
Chromatography
This standard is issued under the fixed designation E 840; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope CGA P-1 Safe Handling of Compressed Gases in Contain-
ers
1.1 This practice is intended as a guide for the use of a flame
CGA G-5.4 Standard for Hydrogen Piping Systems at
photometric detector (FPD) as the detection component of a
Consumer Locations
gas chromatographic system.
CGA P-9 The Inert Gases: Argon, Nitrogen and Helium
1.2 This practice is directly applicable to an FPD that
CGA V-7 Standard Method of Determining Cylinder Valve
employs a hydrogen-air flame burner, an optical filter for
Outlet Connections for Industrial Gas Mixtures
selective spectral viewing of light emitted by the flame, and a
CGA P-12 Safe Handling of Cryogenic Liquids
photomultiplier tube for measuring the intensity of light
HB-3 Handbook of Compressed Gases
emitted.
1.3 This practice describes the most frequent use of the FPD
3. Terminology
which is as an element-specific detector for compounds con-
3.1 Definitions—For definitions relating to gas chromatog-
taining sulfur (S) or phosphorus (P) atoms. However, nomen-
raphy, refer to Practice E 355.
clature described in this practice are also applicable to uses of
3.2 Descriptions of Terms—Descriptions of terms used in
the FPD other than sulfur or phosphorus specific detection.
this practice are included in Sections 7-17.
1.4 This practice is intended to describe the operation and
3.3 Symbols:Symbols—A list of symbols and associated
performance of the FPD itself independently of the chromato-
units of measurement is included in Annex A1.
graphic column. However, the performance of the detector is
described in terms which the analyst can use to predict overall
4. Hazards
system performance when the detector is coupled to the
4.1 Gas Handling Safety—The safe handling of com-
column and other chromatographic system components.
pressed gases and cryogenic liquids for use in chromatography
1.5 For general gas chromatographic procedures, Practice
is the responsibility of every laboratory. The Compressed Gas
E 260 should be followed except where specific changes are
Association, (CGA), a member group of specialty and bulk gas
recommended herein for use of an FPD.
suppliers, publishes the following guidelines to assist the
1.6 This standard does not purport to address all of the
laboratory chemist to establish a safe work environment.
safety concerns, if any, associated with its use. It is the
Applicable CG publications include CGA P-1, CGA G-5.4,
responsibility of the user of this standard to establish appro-
CGA P-9, CGA V-7, CGA P-12, and HB-3.
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. For specific safety
5. Principles of Flame Photometric Detectors
information, see Section 4, Hazards.
5.1 The FPD detects compounds by burning those com-
pounds in a flame and sensing the increase of light emission
2. Referenced Documents
from the flame during that combustion process. Therefore, the
2.1 ASTM Standards:
2 FPD is a flame optical emission detector comprised of a
E 260 Practice for Packed Column Gas Chromatography
hydrogen-air flame, an optical window for viewing emissions
E 355 Practice for Gas Chromatography Terms and Rela-
2 generated in the flame, an optical filter for spectrally selecting
tionships
the wavelengths of light detected, a photomultiplier tube for
2.2 CGA Standards:
measuring the intensity of light emitted, and an electrometer
for measuring the current output of the photomultiplier.
5.2 The intensity and wavelength of light emitted from the
FPD flame depends on the geometric configuration of the flame
This practice is under the jurisdiction of ASTM Committee E13 on Molecular
Spectrography and is the direct responsibility of Subcommittee E13.19 on Chro-
burner and on the absolute and relative flow rates of gases
matography.
Current edition approved May 15, 1995. Published July 1995. Originally
published as E 840 – 81. Last previous edition E 840 – 91.
Available from Compressed Gas Association, Inc., 1725 Jefferson Davis
Annual Book of ASTM Standards, Vol 14.02.
Highway, Arlington, VA 22202-4100.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
E 840
supplied to the detector. By judicious selection of burner response depends on the P-atom or S-atom mass flow per unit
geometry and gas flow rates, the FPD flame is usually designed time into the detector, the FPD is a mass flow rate type of
to selectively enhance optical emissions from certain types of detector. The upper limit to the intensity of light emitted from
molecules while suppressing emissions from other molecules. both the HPO and S molecules is generally determined by the
5.3 Typical FPD flames are normally not hot enough to onset of self-absorption effects in the emitting flame. At high
promote abundant optical emissions from atomic species in the concentrations of S and P atoms in the flame, the concentra-
flame. Instead, the optical emissions from an FPD flame tions of ground state S and HPO molecules becomes sufficient
usually are due to molecular band emissions or continuum to reabsorb light emitted from the radiating states of HPO and
emissions resulting from recombination of atomic or molecular S .
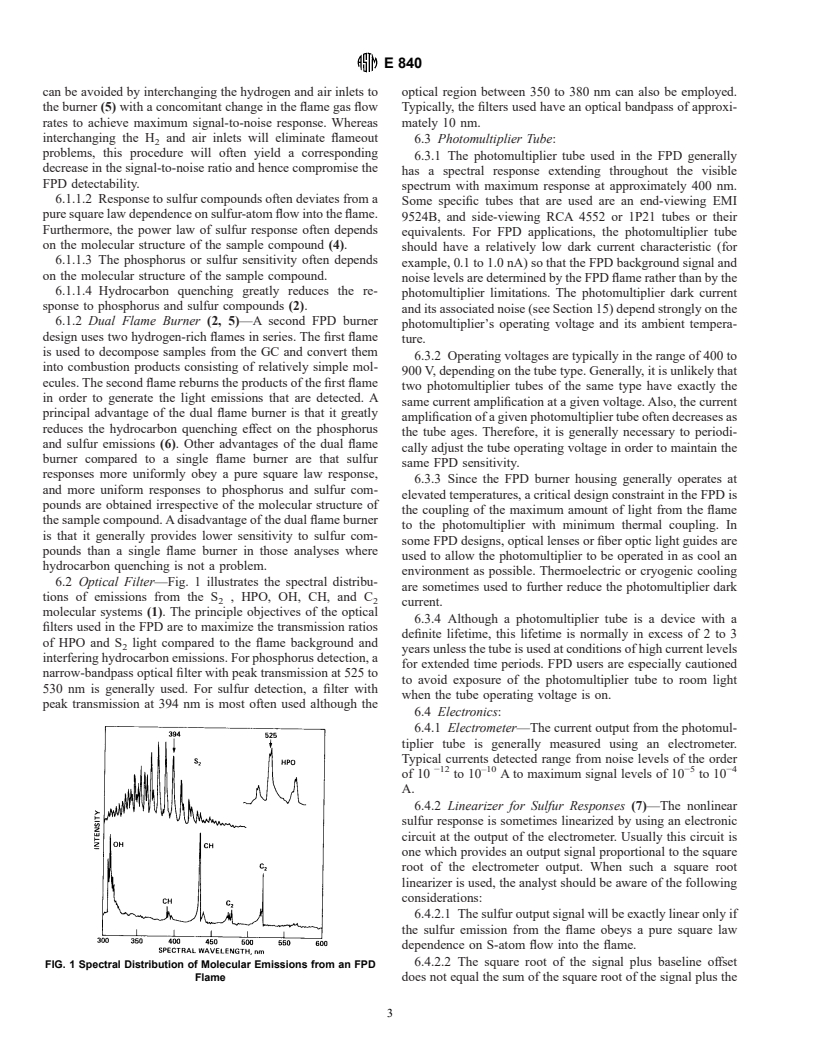
species in the flame. For sulfur detection, light emanating from 5.6 In the presence of a hydrocarbon background in the FPD
the S molecule is generally detected. For phosphorus detec- flame, the light emissions from the phosphorus and sulfur
tion, light emanating from the HPO molecule is generally compounds can be severely quenched (2). Such quenching can
detected. Interfering light emissions from general hydrocarbon occur in the gas chromatographic analysis of samples so
compounds are mainly comprised of CH and C molecular complex that the GC column does not completely separate the
band emissions, and CO + O → CO +hg continuum radia- phosphorus or sulfur compounds from overlapping hydrocar-
tion. bon compounds. Quenching can also occur as the result of an
5.4 Hydrogen – air or hydrogen – oxygen diffusion flames underlying tail of a hydrocarbon solvent peak preceding
are normally employed for the FPD. In such diffusion flames, phosphorus or sulfur compounds in a chromatographic sepa-
the hydrogen and oxygen do not mix instantaneously, so that ration. The fact that the phosphorus or sulfur response is
these flames are characterized by significant spatial variations reduced by quenching is not always apparent from a chromato-
in both temperature and chemical species. The important gram since the FPD generally gives little response to the
chemical species in a hydrogen – air flame are the H, O, and hydrocarbon. The existence of quenching can often be revealed
OH flame radicals. These highly reactive species play a major by a systematic investigation of the variation of the FPD
role in decomposing incoming samples and in the subsequent response as a function of variations in sample volume while the
production of the desired optical emissions. Optical emissions analyte is held at a constant amount.
from the HPO and S molecular systems are highly favored in 5.7 The chromatographic detection of trace level phospho-
those regions of an FPD flame which are locally rich in rus or sulfur compounds can be complicated by the fact that
H-atoms, while CH and C light emissions from hydrocarbons such compounds often tend to be highly reactive and adsorp-
originate mainly from those flame regions which are locally tive. Therefore, care must be taken to ensure that the entire
rich in O-atoms. The highest sensitivity and specificity for chromatographic system is properly free of active sites for
sulfur and phosphorus detection are achieved only when the adsorption of phosphorus or sulfur compounds. The use of
FPD flame is operated with hydrogen in excess of that silanized glass tubing as GC injector liners and GC column
stoichiometric amount required for complete combustion of the materials is a good general practice. At near ambient tempera-
oxygen supplied to the flame. This assures a large flame tures, GC packed columns made of FEP TFE-fluorocarbon,
volume that is locally abundant in H-atoms, and a minimal specially coated silica gel, or treated graphitized carbon are
flame volume that is locally abundant in O-atoms. The sensi- often used for the analysis of sulfur gases.
tivity and specificity of the FPD are strongly dependent on the
6. Detector Construction
absolute and relative flow rates of hydrogen and air. The
6.1 Burner Design:
optimum hydrogen and air flow rates depend on the detailed
6.1.1 Single Flame Burner (2,3)—The most popular FPD
configuration of the flame burner. For some FPD designs, the
burner uses a single flame to decompose sample compounds
flows which are optimum for phosphorus detection are not the
and generate the optical emissions. In this burner, carrier gas
same as the flows which are optimum for sulfur detection.
and sample compounds in the effluent of a GC column are
Also, the flows which are optimum for one sample compound
mixed with air and conveyed to an orifice in the center of a
may not necessarily be optimum for another sample com-
flame tip. Excess hydrogen is introduced from the outer
pound.
perimeter of this flame tip so as to produce a relatively large,
5.5 Although the detailed chemistry occurring in the FPD
diffuse hydrogen-rich flame. With this burner and flow con-
flame has not been firmly established, it is known that the
figuration, light emissions from hydrocarbon compounds occur
intense emissions from the HPO and S molecules are the result
primarily in the locally oxygen-rich core of the flame in close
of chemiluminescent reactions in the flame rather than thermal
proximity to the flame tip orifice, while HPO and S emissions
excitation of these molecules (1). The intensity of light 2
occur primarily in the upper hydrogen-rich portions of the
radiated from the HPO molecule generally varies as a linear
flame. Improved specificity is therefore obtained by the use of
function of P-atom flow into the flame. In the case of the S
an optical shield at the base of the flame to prevent hydrocar-
emission, the light intensity is generally a nonlinear function of
bon emissions from being in the direct field of view. The light
S-atom flow into the flame, and most often is found to vary as
emissions generated in this flame are generally viewed from
the approximate square of the S-atom flow. Since the FPD
the side of the flame. Some of the known limitations of this
burner are as follows:
6.1.1.1 Solvent peaks in the GC effluent can momentarily
The boldface numerals in parentheses refer to the list of references at the end
of this practice. starve the flame of oxygen and cause a flameout. This effect
E 840
can be avoided by interchanging the hydrogen and air inlets to optical region between 350 to 380 nm can also be employed.
the burner (5) with a concomitant change in the flame gas flow Typically, the filters used have an optical bandpass of approxi-
rates to achieve maximum signal-to-noise response. Whereas mately 10 nm.
interchanging the H and air inlets will eliminate flameout
6.3 Photomultiplier Tube:
problems, this procedure will often yield a corresponding
6.3.1 The photomultiplier tube used in the FPD generally
decrease in the signal-to-noise ratio and hence compromise the
has a spectral response extending throughout the visible
FPD detectability.
spectrum with maximum response at approximately 400 nm.
6.1.1.2 Response to sulfur compounds often deviates from a
Some specific tubes that are used are an end-viewing EMI
pure square law dependence on sulfur-atom flow into the flame.
9524B, and side-viewing RCA 4552 or 1P21 tubes or their
Furthermore, the power law of sulfur response often depends
equivalents. For FPD applications, the photomultiplier tube
on the molecular structure of the sample compound (4).
should have a relatively low dark current characteristic (for
6.1.1.3 The phosphorus or sulfur sensitivity often depends
example, 0.1 to 1.0 nA) so that the FPD background signal and
on the molecular structure of the sample compound.
noise levels are determined by the FPD flame rather than by the
6.1.1.4 Hydrocarbon quenching greatly reduces the re-
photomultiplier limitations. The photomultiplier dark current
sponse to phosphorus and sulfur compounds (2).
and its associated noise (see Section 15) depend strongly on the
6.1.2 Dual Flame Burner (2, 5)—A second FPD burner
photomultiplier’s operating voltage and its ambient tempera-
design uses two hydrogen-rich flames in series. The first flame
ture.
is used to decompose samples from the GC and convert them
6.3.2 Operating voltages are typically in the range of 400 to
into combustion products consisting of relatively simple mol-
900 V, depending on the tube type. Generally, it is unlikely that
ecules. The second flame reburns the products of the first flame
two photomultiplier tubes of the same type have exactly the
in order to generate the light emissions that are detected. A
same current amplification at a given voltage. Also, the current
principal advantage of the dual flame burner is that it greatly
amplification of a given photomultiplier tube often decreases as
reduces the hydrocarbon quenching effect on the phosphorus
the tube ages. Therefore, it is generally necessary to periodi-
and sulfur emissions (6). Other advantages of the dual flame
cally adjust the tube operating voltage in order to maintain the
burner compared to a single flame burner are that sulfur
same FPD sensitivity.
responses more uniformly obey a pure square law response,
6.3.3 Since the FPD burner housing generally operates at
and more uniform responses to phosphorus and sulfur com-
elevated temperatures, a critical design constraint in the FPD is
pounds are obtained irrespective of the molecular structure of
the coupling of the maximum amount of light from the flame
the sample compound. A disadvantage of the dual flame burner
to the photomultiplier with minimum thermal coupling. In
is that it generally provides lower sensitivity to sulfur com-
some FPD designs, optical lenses or fiber optic light guides are
pounds than a single flame burner in those analyses where
used to allow the photomultiplier to be operated in as cool an
hydrocarbon quenching is not a problem.
environment as p
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.