ASTM E2111-05
(Test Method)Standard Quantitative Carrier Test Method to Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and Sporicidal Potencies of Liquid Chemical Microbicides
Standard Quantitative Carrier Test Method to Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and Sporicidal Potencies of Liquid Chemical Microbicides
SIGNIFICANCE AND USE
This test method is fully quantitative and it also avoids any loss of viable organisms through wash off. This makes it possible to produce statistically valid data using many fewer test and control carriers than other quantitative methods based on most probable numbers (MPN).
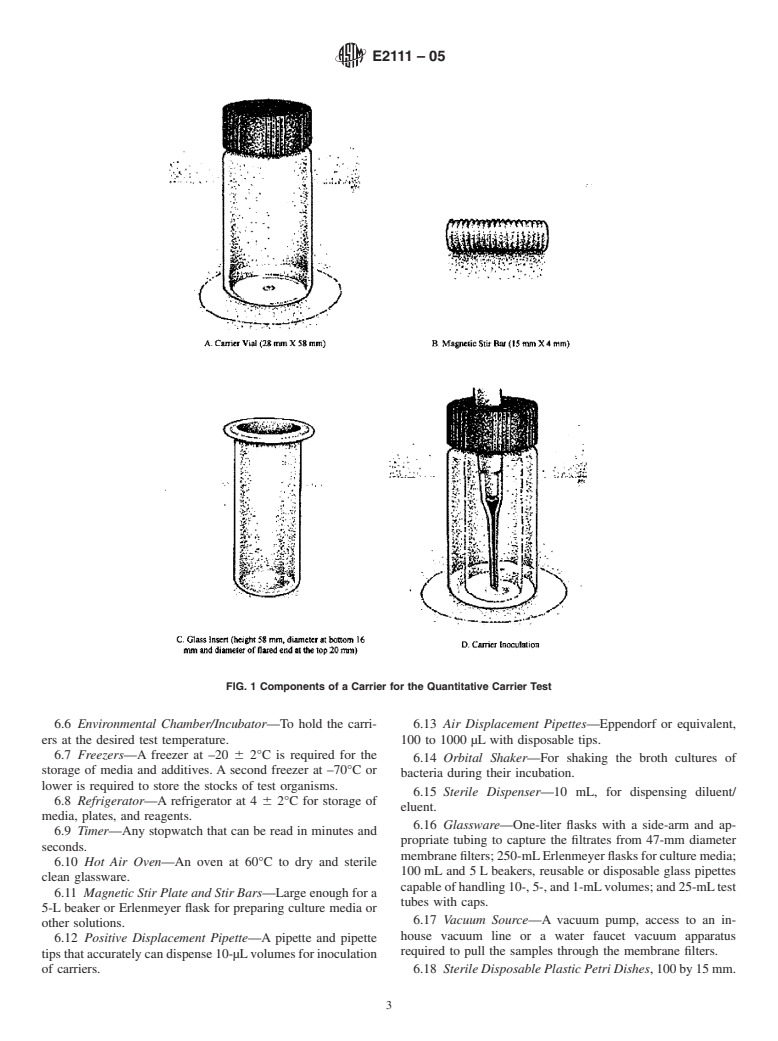
The design of the carriers makes it possible to place into each a precisely measured volume of the test suspension. The use of the threaded stir bars allows for efficient recovery of the inoculum even after its exposure for several hours to strong fixatives such as glutaraldehyde.
The membrane filtration step allows processing of the entire eluate from the test carriers and therefore the capture and subsequent detection of even low numbers of viable organisms that may be present.
This test can be performed with or without a soil load to determine the effect of such loading on microbicide performance. The soil load developed for this test is a mixture of three types of proteins (high molecular weight proteins, low molecular weight peptides, and mucous material) to represent the body secretions, excretions, or other extraneous substances that chemical microbicides may encounter under field conditions. It is suitable for working with the various test organisms included here. The components of the soil load are readily available and subject to much less variability than animal sera.
Since the quality of tap water varies considerably both geographically and temporally, this test method incorporates the use of water with a specified and documented level of hardness to prepare use-dilutions of test products. The U.S. Environmental Protection Agency’Scientific Advisory Panel (SAP) on Germicide Test Methodology has recommended the use of water with a standard hardness of 400 ppm as CaCO3.
SCOPE
1.1 This test method is designed for use in product development and for the generation of product potency data. This test method permits the loading of each carrier with a known volume of the test organism. The incorporation of controls can also determine the initial load of colony forming units (CFU) of organisms on the test carriers and any loss in CFU after the mandatory drying of the inoculum.
1.2 This test method is designed to have survivors and also to be used with a performance standard. The surviving microorganisms on each test carrier are compared to the mean of no less than three control carriers to determine if the performance standard has been met. To allow proper statistical evaluation of results, the size of the test inoculum should be sufficiently large to take into account both the performance standard and the experimental variation in the results. For example, if an arbitrary performance standard of 6-log10 reduction in the viability titer of the test organism is used, and an inoculum size of 107 CFU, then theoretically a maximum of ten survivors per carrier is permitted; however, because of experimental variability, the exact target may need to be higher than 106 CFU/carrier, thus fewer survivors would be permitted.
1.3 This test method should be performed by persons with training in microbiology and in facilities designed and equipped for work with infectious agents at the appropriate biosafety level (3).
1.4 In this test method, SI units are used for all applications, except for distance, in which case inches are used and SI units follow.
1.5 It is the responsibility of the investigator to determine whether Good Laboratory Practice Regulations (GLPs) are required and to follow them where appropriate (40 CFR, Part 160 for EPA submissions and 21 CFR, Part 58 for FDA submissions).
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E2111–05
Standard Quantitative Carrier Test Method to
Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and
1
Sporicidal Potencies of Liquid Chemical Microbicides
This standard is issued under the fixed designation E2111; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
2
The need for better tests to assess the microbicidal activity of chemicals was recognized (1) and
several simpler and quantitative test methods have been developed for working with a wide variety of
microorganisms (2). The test method described here uses glass vials as carriers; the same basic set of
materials and procedures can be used to test the potency of liquid microbicides against vegetative
bacteria,fungi,mycobacteria,andbacterialspores.However,thetestmethodisnotappropriateforuse
with viruses because of the relatively high levels of eluate dilutions required and the need for
membrane filtration. Further evaluation of products under more stringent test conditions may be
necessary for their registration. Performance standards for the categories of products to be tested and
the specific types of organism(s) to be used may also vary depending on the regulatory agency.
1. Scope 1.3 This test method should be performed by persons with
training in microbiology and in facilities designed and
1.1 This test method is designed for use in product devel-
equipped for work with infectious agents at the appropriate
opment and for the generation of product potency data. This
biosafety level (3).
test method permits the loading of each carrier with a known
1.4 In this test method, SI units are used for all applications,
volume of the test organism. The incorporation of controls can
except for distance, in which case inches are used and SI units
also determine the initial load of colony forming units (CFU)
follow.
of organisms on the test carriers and any loss in CFU after the
1.5 It is the responsibility of the investigator to determine
mandatory drying of the inoculum.
whether Good Laboratory Practice Regulations (GLPs) are
1.2 This test method is designed to have survivors and also
required and to follow them where appropriate (40 CFR, Part
to be used with a performance standard. The surviving micro-
160 for EPA submissions and 21 CFR, Part 58 for FDA
organisms on each test carrier are compared to the mean of no
submissions).
less than three control carriers to determine if the performance
1.6 This standard does not purport to address all of the
standard has been met.To allow proper statistical evaluation of
safety concerns, if any, associated with its use. It is the
results,thesizeofthetestinoculumshouldbesufficientlylarge
responsibility of the user of this standard to establish appro-
to take into account both the performance standard and the
priate safety and health practices and determine the applica-
experimental variation in the results. For example, if an
bility of regulatory limitations prior to use.
arbitrary performance standard of 6-log reduction in the
10
viability titer of the test organism is used, and an inoculum size
2. Referenced Documents
7
of 10 CFU, then theoretically a maximum of ten survivors per
3
2.1 ASTM Standards:
carrier is permitted; however, because of experimental vari-
6
D1129 Terminology Relating to Water
ability, the exact target may need to be higher than 10
D1193 Specification for Reagent Water
CFU/carrier, thus fewer survivors would be permitted.
E1054 Test Methods for Evaluation of Inactivators of An-
timicrobial Agents
1
This test method is under the jurisdiction of ASTM Committee E35 on
2.2 CFR Standards:
Pesticides and Alternative Control Agents and is the direct responsibility of
Subcommittee E35.15 on Antimicrobial Agents.
Current edition approved Nov. 1, 2005. Published December 2005. Originally
3
approved in 2000. Last previous edition approved in 2000 as E2111 – 00. DOI: For referenced ASTM standards, visit the ASTM website, www.astm.org, or
10.1520/E2111–05. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
2
The boldface numbers in parentheses refer to the list of references at the end of Standards volume information, refer to the standard’s Document Summary page on
this standard. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
E2111–05
4
40 CFR, Part 160 ate recovery medium in a 100-mm diameter petri plate. The
4
21 CFR, Part 58 plates are held for the required period at the desired incubation
t
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.