ASTM E2111-12(2018)
(Test Method)Standard Quantitative Carrier Test Method to Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and Sporicidal Potencies of Liquid Chemicals
Standard Quantitative Carrier Test Method to Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and Sporicidal Potencies of Liquid Chemicals
SIGNIFICANCE AND USE
5.1 This test method is fully quantitative and it also avoids any loss of viable organisms through wash off, making it possible to produce statistically valid data using many fewer test and control carriers than other quantitative methods based on most probable numbers (MPN).
5.2 The design of the carriers makes it possible to place into each a precisely measured volume of the test suspension. The use of the threaded stir bars allows for efficient recovery of the inoculum even after its exposure for several hours to strong fixatives such as glutaraldehyde.
5.3 The membrane filtration step allows processing of the entire eluate from the test carriers and therefore the capture and subsequent detection of even low numbers of viable organisms that may be present.
5.4 This test can be performed with or without a soil load to determine the effect of such loading on microbicide performance. Consult the target regulatory agency on the need, type(s), and acceptable level(s) of soil load prior to testing. One type of soil load (Quantitative Disk Carrier Test Method E2197) to consider for this test is a mixture of three types of proteins (high molecular weight proteins, low molecular weight peptides, and mucous material) to represent the body secretions, excretions, or other extraneous substances that chemical microbicides may encounter under field conditions. It is suitable for working with the various test organisms included here. The components of the soil load are readily available and subject to much less variability than animal sera.
5.5 If distilled water or other diluent is not to be specified on the product label, the diluent for the test substance is assumed to be tap water. Since the quality of tap water varies considerably both geographically and temporally, this test method incorporates the use of water with a specified and documented level of hardness to prepare use-dilutions of test substance that require dilution in water before use. Consult the tar...
SCOPE
1.1 This test method is designed for use in product development and for the generation of product potency data. This test method permits the loading of each carrier with a known volume of the test organism. The incorporation of controls can also determine the initial load of colony forming units (CFU) of organisms on the test carriers and any loss in CFU after the mandatory drying of the inoculum.
1.2 This test method is designed to have survivors and also to be used with a performance standard. The surviving microorganisms on each test carrier are compared to the mean of no less than three control carriers to determine if the performance standard has been met. To allow proper statistical evaluation of results, the size of the test inoculum should be sufficiently large to take into account both the performance standard and the experimental variation in the results. For example, if an arbitrary performance standard of 6-log10 reduction in the viability titer of the test organism is used, and an inoculum size of 107 CFU, then theoretically a maximum of ten survivors per carrier is permitted; however, because of experimental variability, the exact target may need to be higher than 106 CFU/carrier, thus fewer survivors would be permitted.
1.3 This test method should be performed by persons with training in microbiology and in facilities designed and equipped for work with infectious agents at the appropriate biosafety level (3).
1.4 In this test method, SI units are used for all applications, except for distance, in which case inches are used and SI units follow.
1.5 It is the responsibility of the investigator to determine whether Good Laboratory Practice Regulations (GLPs) are required and to follow them where appropriate (40 CFR Part 160 for EPA submissions and 21 CFR Part 58 for FDA submissions).
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It ...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E2111 − 12 (Reapproved 2018)
Standard Quantitative Carrier Test Method to
Evaluate the Bactericidal, Fungicidal, Mycobactericidal, and
Sporicidal Potencies of Liquid Chemicals
This standard is issued under the fixed designation E2111; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
The need for better tests to assess the microbicidal activity of chemicals was recognized (1) and
severalsimplerandquantitativetestmethodshavebeendevelopedforworkingwithawidevarietyof
microorganisms (2). The test method described here uses glass vials as carriers; the same basic set of
materials and procedures can be used to test the potency of liquid microbicides against vegetative
bacteria,fungi,mycobacteria,andbacterialspores.However,thetestmethodisnotappropriateforuse
with viruses because of the relatively high levels of eluate dilutions required and the need for
membrane filtration. Further evaluation of products under more stringent test conditions may be
necessary for their registration. Performance standards for the categories of products to be tested and
the specific types of organism(s) to be used may also vary depending on the regulatory agency.
1. Scope carrier is permitted; however, because of experimental
variability, the exact target may need to be higher than 10
1.1 This test method is designed for use in product devel-
CFU/carrier, thus fewer survivors would be permitted.
opment and for the generation of product potency data. This
test method permits the loading of each carrier with a known
1.3 This test method should be performed by persons with
volume of the test organism.The incorporation of controls can training in microbiology and in facilities designed and
also determine the initial load of colony forming units (CFU)
equipped for work with infectious agents at the appropriate
of organisms on the test carriers and any loss in CFU after the biosafety level (3).
mandatory drying of the inoculum.
1.4 Inthistestmethod,SIunitsareusedforallapplications,
1.2 This test method is designed to have survivors and also
except for distance, in which case inches are used and SI units
to be used with a performance standard. The surviving micro-
follow.
organisms on each test carrier are compared to the mean of no
1.5 It is the responsibility of the investigator to determine
less than three control carriers to determine if the performance
whether Good Laboratory Practice Regulations (GLPs) are
standardhasbeenmet.Toallowproperstatisticalevaluationof
required and to follow them where appropriate (40 CFR Part
results,thesizeofthetestinoculumshouldbesufficientlylarge
160 for EPA submissions and 21 CFR Part 58 for FDA
to take into account both the performance standard and the
submissions).
experimental variation in the results. For example, if an
1.6 This standard does not purport to address all of the
arbitrary performance standard of 6-log reduction in the
safety concerns, if any, associated with its use. It is the
viabilitytiterofthetestorganismisused,andaninoculumsize
responsibility of the user of this standard to establish appro-
of10 CFU,thentheoreticallyamaximumoftensurvivorsper
priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
This test method is under the jurisdiction of ASTM Committee E35 on
1.7 This international standard was developed in accor-
Pesticides, Antimicrobials, and Alternative Control Agents and is the direct
dance with internationally recognized principles on standard-
responsibility of Subcommittee E35.15 on Antimicrobial Agents.
Current edition approved Sept. 15, 2018. Published March 2019. Originally
ization established in the Decision on Principles for the
approved in 2000. Last previous edition approved in 2012 as E2111–12. DOI:
Development of International Standards, Guides and Recom-
10.1520/E2111–12R18.
2 mendations issued by the World Trade Organization Technical
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
this standard. Barriers to Trade (TBT) Committee.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2111 − 12 (2018)
2. Referenced Documents a soil load. The contamination of the inside surface of the
3 carrier with microaerosols is avoided by the use of glass
2.1 ASTM Standards:
inserts. The inoculum is dried and exposed to 1 mLof the test
D1129Terminology Relating to Water
microbicide for the desired contact time at the recommended
D1193Specification for Reagent Water
temperature; control carriers receive 1 mL of normal saline
E1054Test Methods for Evaluation of Inactivators of Anti-
instead. At the end of the contact time, 9 mL of an eluent
microbial Agents
without or with a neutralizer, is added to the vial to dilute/
E2197Quantitative Disk Carrier Test Method for Determin-
neutralize the microbicide and any inoculum adhering to the
ing Bactericidal, Virucidal, Fungicidal, Mycobactericidal,
carrier surface is recovered using a magnetic stir bar with a
and Sporicidal Activities of Chemicals
threaded surface. The eluate is passed through a membrane
E2756Terminology Relating toAntimicrobial andAntiviral
filter, the carrier vial is then rinsed several times with eluent/
Agents
4 diluent and the rinses are also passed through the same filter.
2.2 CFR Standards:
Thetotalrinsevolumeisnolessthan100mL.Controlandtest
40 CFRPart 160
eluates requiring dilution to get countable colonies are first
21 CFRPart 58
subjected to a series of tenfold dilutions and the material from
suitable dilutions is passed separately through membrane
3. Terminology
filters. Each filter is placed on the agar surface of an appropri-
3.1 Definitions of Terms Specific to This Standard:
ate recovery medium in a 100-mm diameter petri plate. The
3.1.1 carrier,n—inanimatesurfaceorobjectinoculatedwith
platesareheldfortherequiredperiodatthedesiredincubation
the test organism.
temperature, colonies counted, and log reductions in the
3.1.2 eluate, n—eluent,whichcontainstherecoveredorgan-
viability titer of the test organism calculated.
ism(s).
NOTE1—Donotsoakthemagneticstirbarsinethanolorothersolvents
for decontamination as this may damage the sealant on them.
3.1.3 eluent, n—any solution that is harmless to the test
organism(s) and that is added to a carrier to recover the
5. Significance and Use
organism(s) in or on it.
5.1 This test method is fully quantitative and it also avoids
3.1.4 neutralization, n—processtoquenchtheantimicrobial
any loss of viable organisms through wash off, making it
activityofatestformulation.Thisprocessmaybeachievedby
possible to produce statistically valid data using many fewer
dilution of the organism/test formulation mixture and/or by
test and control carriers than other quantitative methods based
adding to it one or more chemical neutralizers. (Refer to Test
on most probable numbers (MPN).
Methods E1054 for further details
3.1.4.1 Discussion—This process may be achieved by dilu-
5.2 Thedesignofthecarriersmakesitpossibletoplaceinto
tionoftheorganism/testformulationmixtureorbyaddingtoit
each a precisely measured volume of the test suspension. The
one or more chemical neutralizers, or both.
use of the threaded stir bars allows for efficient recovery of the
3.1.5 soil load, n—solution of one or more organic, or inoculum even after its exposure for several hours to strong
fixatives such as glutaraldehyde.
inorganic substances, or both, added to the suspension of the
test organism to simulate the presence of body secretions,
5.3 The membrane filtration step allows processing of the
excretions, or other extraneous substances.
entireeluatefromthetestcarriersandthereforethecaptureand
3.1.6 test formulation, n—formulation that incorporates an-
subsequentdetectionofevenlownumbersofviableorganisms
timicrobial ingredients.
that may be present.
3.1.7 test organism, n—applied inoculum of an organism
5.4 Thistestcanbeperformedwithorwithoutasoilloadto
that has characteristics that allows it to be readily identified. It
determine the effect of such loading on microbicide perfor-
also may be referred to as a surrogate or a marker organism.
mance. Consult the target regulatory agency on the need,
type(s),andacceptablelevel(s)ofsoilloadpriortotesting.One
4. Summary of Test Method
type of soil load (Quantitative Disk Carrier Test Method
4.1 This is a fully quantitative carrier test method suitable
E2197) to consider for this test is a mixture of three types of
for assessing the potency of chemicals against vegetative
proteins (high molecular weight proteins, low molecular
bacteria, fungi, mycobacteria, as well as bacterial spores. It is
weight peptides, and mucous material) to represent the body
designed primarily for testing formulations to be used on hard
secretions, excretions, or other extraneous substances that
environmental surfaces and medical devices. This test method
chemicalmicrobicidesmayencounterunderfieldconditions.It
uses the flat inside bottom surface of glass vials as the carrier.
issuitableforworkingwiththevarioustestorganismsincluded
Each vial receives 10 µL of the test organism with or without
here.Thecomponentsofthesoilloadarereadilyavailableand
subject to much less variability than animal sera.
5.5 Ifdistilledwaterorotherdiluentisnottobespecifiedon
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
the product label, the diluent for the test substance is assumed
Standards volume information, refer to the standard’s Document Summary page on
to be tap water. Since the quality of tap water varies consid-
the ASTM website.
erably both geographically and temporally, this test method
AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401. incorporates the use of water with a specified and documented
E2111 − 12 (2018)
level of hardness to prepare use-dilutions of test substance that 6.4 Filter Sterilization System for Media and Reagents—A
require dilution in water before use. Consult the target regula- membrane or cartridge filtration system (0.22-µm pore diam-
toryagencyregardingtheuseandlevelofwaterhardnessprior eter) is required for sterilizing heat-sensitive solutions.
to testing.
6.5 Membrane Filtration System for Capture of the Test
Organisms—Sterile 47-mm diameter sterilizing membrane fil-
6. General Equipment and Labware
ters and glass, metal, or plastic holders for such filters are
6.1 Laminar Flow Cabinet—AClass II (TypeA) biological
required. Membranes made from polyethersulfone (PES) are
safety cabinet for this work. The procedures for the proper
recommended. Filter membranes with a pore diameter of 0.22
maintenance and use of such cabinets are given in Ref 3.
µm must be used when working with bacterial spores.
6.2 Incubator—An ordinary incubator and an anaerobic
6.6 Environmental Chamber/Incubator—To hold the carri-
incubator. If only one ordinary incubator is available, its
ers at the desired test temperature.
temperature will require adjustment depending on the type of
6.7 Freezers—A freezer at –20 6 2°C is required for the
organism under test.
storage of media and additives. A second freezer at –70°C or
6.3 Sterilizer—Any steam sterilizer suitable for processing
lower is required to store the stocks of test organisms.
culture media, reagents and labware is acceptable. The steam
supplied to the sterilizer must be free from additives toxic to 6.8 Refrigerator—A refrigerator at 4 6 2°C for storage of
the test organisms. media, plates, and reagents.
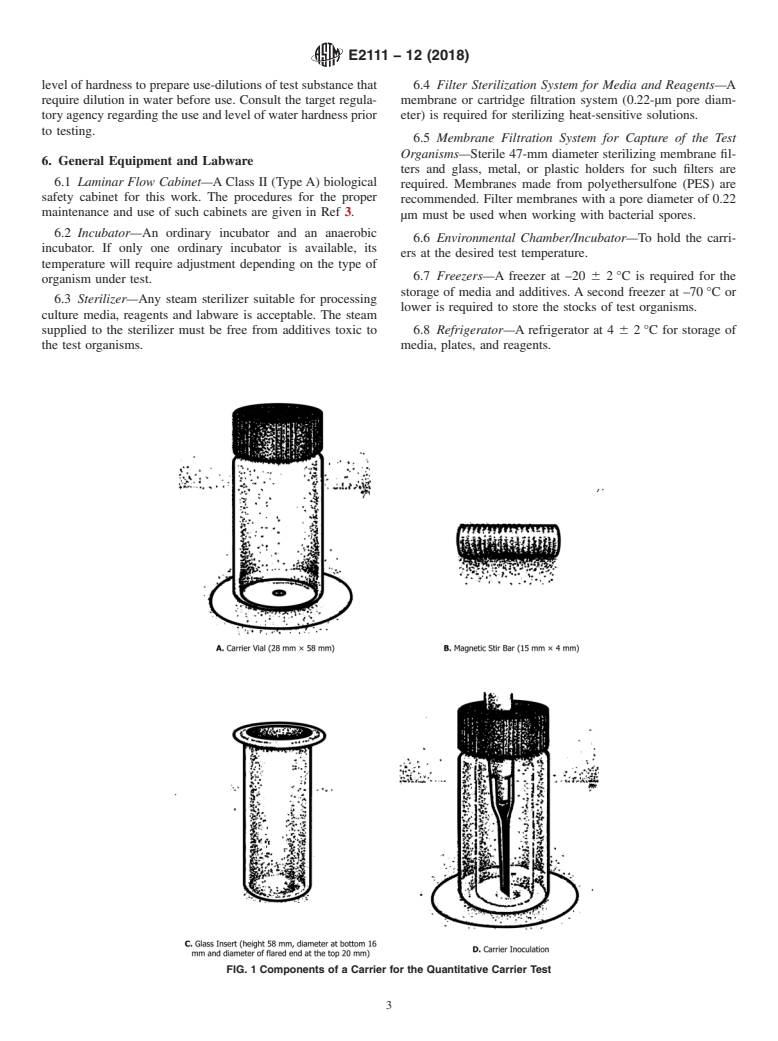
FIG. 1 Components of a Carrier for the Quantitative Carrier Test
E2111 − 12 (2018)
6.9 Timer—Any stopwatch that can be read in minutes and 6.25 Aluminum Foil, to wrap items to be sterilized.
seconds.
6.26 Vortex Mixer, to vortex the eluate and rinsing fluid in
6.10 Hot Air Oven—An oven at 60°C to dry and sterile the carrier to ensure efficient recovery of the test organism(s).
clean glassware.
6.27 Glass Inserts, to be placed inside the glass carriers
6.11 Magnetic Stir Plate and Stir Bars—Large enough for a during inoculation with the test organism. Such inserts have
5-Lbeaker or Erlenmeyer flask for preparing culture media or been found to eliminate the deposition of microaerosols on the
other solutions. inside walls of the carriers. Glass inserts may be manufactured
according to Fig. 1C.
6.12 Positive Displacement Pipette—A pipette and pipette
tipsthataccuratelycandispense10-µLvolumesforinoculation 6.28 Centrifuge, for concentration, or washing, or both of
of carriers. the cells/spores of the test organism(s).
6.13 Air Displacement Pipettes—Eppendorf or equivalent, 6.29 Markers, permanent labware marking pens.
100 to 1000 µL with disposable tips.
6.30 Sterile Polypropylene Centrifuge Tubes with Caps, 50
6.14 Orbital Shaker—For shaking the broth cultures of mL.
bacteria during their incubation.
6.31 Colony Counter,forexample,QuebecColonyCounter.
6.15 Sterile Dispenser—10 mL, for dispensing diluent/
6.32 Sterile Disposable Gloves, for handling the carriers.
eluent.
6.33 Hemocytometer, for counting fungal conidia.
6.16 Glassware—One-liter flasks with a side-arm and ap-
6.34 Spectrophotometer, for measuring turbidity of micro-
propriate tubing to capture the filtrates from 47-mm diameter
bial suspensions.
membranefilters;250-mLErlenmeyerflasksforculturemedia;
100mL and 5L beakers, reusable or disposable glass pipettes
6.35 Bunsen Burner, for aseptic technique
capableofhandling10-,5-,and1-mLvolumes;and25-mLtest
7. General Solutions and Reagents
tubes with caps.
7.1 Purity of Reagents—Reagent grade chemicals shall be
6.17 Vacuum Source—A vacuum pump, access to an in-
used in all tests. Unless otherwise indicated, it is intended that
house vacuum line or a water faucet vacuum apparatus
all reagents conform to the specifications of the Committee on
required to pull the samples through the membrane filters.
Analytical Reagents of the American Chemical Society (4).
6.18 Sterile Disposable Plastic Petri Dishes,100by15mm.
Other grades may be used (5), provided it is first ascertained
6.19 Forceps,straightorcurved,withsmoothtipstohandle that the reagent is of sufficiently high purity
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.