ASTM D4084-94(1999)
(Test Method)Standard Test Method for Analysis of Hydrogen Sulfide in Gaseous Fuels (Lead Acetate Reaction Rate Method)
Standard Test Method for Analysis of Hydrogen Sulfide in Gaseous Fuels (Lead Acetate Reaction Rate Method)
SCOPE
1.1 This test method covers the determination of hydrogen sulfide (H 2 S) in gaseous fuels. It is applicable to the measurement of H 2 S in natural gas, liquefied petroleum gas (LPG), substitute natural gas, and mixtures of fuel gases. Air does not interfere. The applicable range is 0.1 to 16 parts per million by volume (ppm/v) (approximately 0.1 to 22 mg/m ) and may be extended to 100% H 2 S by manual or automatic volumetric dilution.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D 4084 – 94 (Reapproved 1999)
Standard Test Method for
Analysis of Hydrogen Sulfide in Gaseous Fuels (Lead
Acetate Reaction Rate Method)
This standard is issued under the fixed designation D 4084; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope H Sreactswithleadacetatetoformabrownstainonthepaper.
The rate of reaction and resulting rate of color change is
1.1 This test method covers the determination of hydrogen
proportional to the concentration of H S in the sample. An
sulfide (H S) in gaseous fuels. It is applicable to the measure-
optical system, photodetectors, a means to obtain the first
ment of H S in natural gas, liquefied petroleum gas (LPG),
derivativeofthephotodetectorsignal,andameanstoobtainan
substitute natural gas, and mixtures of fuel gases.Air does not
output from the differentiation process comprises the analyzer.
interfere. The applicable range is 0.1 to 16 parts per million by
When there is no change in the color of the tape, and no
volume (ppm/v) (approximately 0.1 to 22 mg/m ) and may be
resulting change in photodetector output, E, the first derivative,
extended to 100 % H S by manual or automatic volumetric
dE/dt, is zero. This results in an analyzer that automatically
dilution.
zeroes when there is no H S.
1.2 This standard does not purport to address all of the 2
safety concerns, if any, associated with its use. It is the
4. Significance and Use
responsibility of the user of this standard to establish appro-
4.1 This test method is useful in determining the concentra-
priate safety and health practices and determine the applica-
tionofhydrogensulfideingaseoussamplestoverifythatlimits
bility of regulatory limitations prior to use.
setforH Sintheproductgasarecompliedwith.Theautomatic
2. Referenced Documents operation of this method allows unattended measurement of
H S concentration.
2.1 ASTM Standards:
D 1193 Specification for Reagent Water
5. Apparatus
D 1914 Practice for Conversion Units and Factors Relating
3 5.1 Volumetric Measuring Devices—a graduated 10-L cyl-
to Sampling and Analysis of Atmospheres
inder (see Fig. 1) having a movable piston for volumetrically
D 2420 Test Method for Hydrogen Sulfide in Liquefied
4 measuring test gas. Gastight syringes of 0.1- and 0.5-mL
Petroleum (LP) Gases (Lead Acetate Method)
volumeforvolumetricallymeasuring100 %H S.Thesegradu-
D 3609 Practice for Calibration Techniques Using Perme-
3 ated devices are not needed if the permeation tube method of
ation Tubes
dynamic mixing is used to prepare the reference sample as this
3. Summary of Test Method method may be used to generate reference mixture.
5.2 Sample Pump—a pump capable of providing more than
3.1 Measurement of H S is accomplished by ratiometrically
8 mL/s (approximately 1 ft /h) at 70 kPa (approximately 10.15
comparing a reading of an unknown sample with that of a
psig). Gas-wetted parts are to be either aluminum or polytet-
known standard sample using a colorimetric analysis method.
rafluorethylene (PTFE). Stainless steel is less preferable but
Pure H S is used as a primary standard and mixed volumetri-
may be used for the purpose of improving safety if applicable.
callywithasulfurfreecarriergasthatisofthesametypeasthe
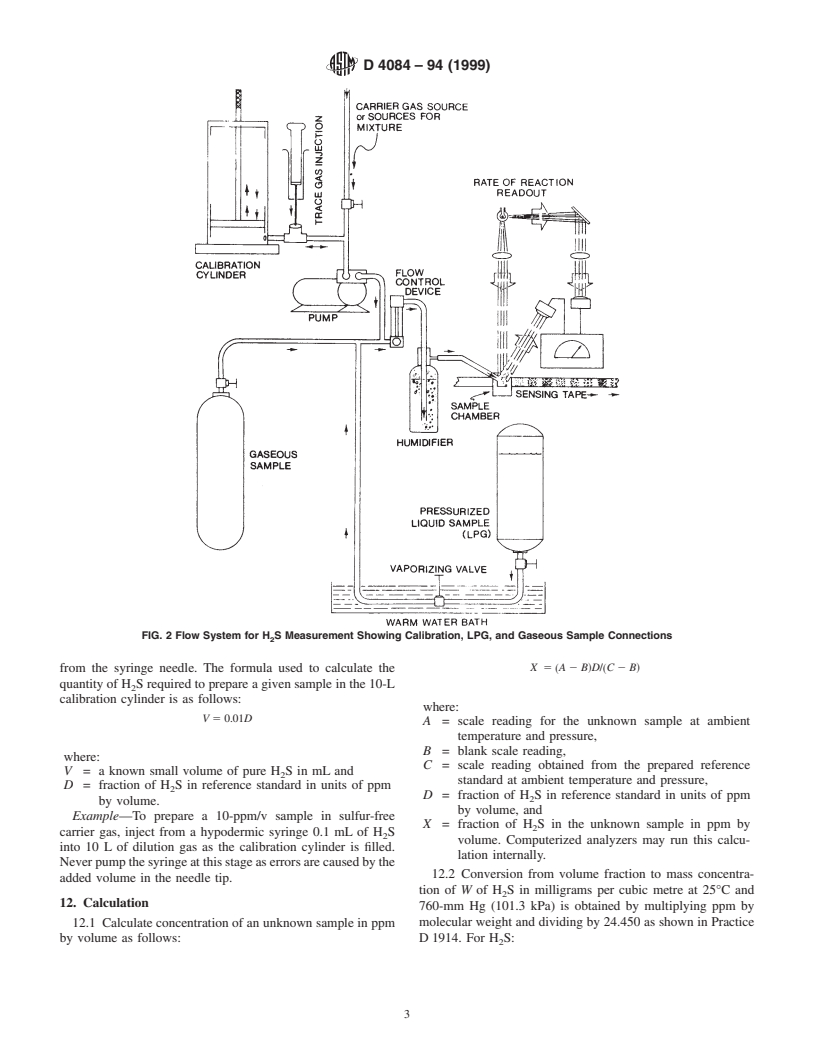
5.3 Colorimetric Rate of Reaction Sensor—select a device
gas to be analyzed. A gaseous sample at constant flow is
of sufficient sensitivity to measure a minimum rate of change
humidified and passed over lead-acetate-impregnated paper.
of color density corresponding to 0.1-ppm H S by volume in
the sample gas. (See Fig. 2.)
This test method is under the jurisdiction ofASTM Committee D-3 on Gaseous
5.4 Recorder, having an adjustable span of 1- to 10-V full
Fuels and is the direct responsibility of Subcommittee D03.05 on Determination of
scale with an input impedance of 1 MV or higher.Aprinter or
Special Constituents of Gaseous Fuels.
Current edition approved Feb. 15, 1994. Published April 1984. Originally
published as D 4084 – 81. Last previous edition D 4084 – 82 (1988).
Annual Book of ASTM Standards, Vol 11.01.
3 5
Annual Book of ASTM Standards, Vol 11.03. A suitable apparatus is available from EnviroTech Controls, Inc., 22001
Annual Book of ASTM Standards, Vol 05.01. Northpark Dr., Kingwood, TX 77339-3804.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 4084 – 94 (1999)
sample, when at atmospheric pressure, is convenient for analysis and will
normally not deteriorate appreciably within 1 h. Slow instrument response
to changes in H S concentration indicates the need for thorough cleaning
of the flow system. (See Appendix X1 for cleaning procedure.) Errors
caused by ambient temperature and pressure changes are compensated by
comparison to a reference standard prepared at the time of analysis.
Preparation of the reference sample is described in Section 11. An
approximatesampleconcentrationisindicatedbytheprocedureinSection
10.
8. Preparation of Instrument
8.1 Fill the humidifier bubbler to the full mark with acetic
acid solution. The acetic acid minimizes interference from
mercaptans. Set the range of the analyzer for the range
FIG. 1 Calibration Sample Preparation Cylinder with Movable
expected in the sample. Connect the pump and set the
Piston
flowmeter for a nominal flow of 8 mL/s (approximately 1
ft /h). Obtain a blank reading by flowing dilution gas through
other output means can be used with digital and computerized
the analyzer. Record the reading of the blank sample as B in
rate of reaction sensors.
12.1. Do not adjust the instrument zero until verification is
obtained, by scrubbing out H S, that the room air or the carrier
6. Reagents and Materials
gas does not contain H S. Absorption on activated charcoal
will remove H S.
6.1 Acetic Acid Solution—Add 50 mL of glacial acid
(CH COOH) to distilled water to make 1 L of solution (5 %).
9. Calibration
Type II distilled water as specified in Specification D 1193 is
satisfactory for the dilution. 9.1 Immediately after having prepared a calibration stan-
dard, obtain its response on the analyzer. Practice D 3609 is
6.2 Hydrogen Sulfide, Lecture Bottle Size—99.5 % by vol-
ume purity or better. An alternative H S source is an H S acceptable as an alternative method for preparation of a
2 2
mixture obtained using permeation tube procedures. Hydrogen reference standard. The analyzer response is recorded as C in
sulfide generated from a solid heated to generate H S may be 12.1.
used instead of the lecture bottle of compressed H S if desired,
10. Sample Measurement Procedure
as a safety precaution.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.