ASTM G5-94(2004)
(Test Method)Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements
Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements
SCOPE
1.1 This test method covers an experimental procedure for checking experimental technique and instrumentation. If followed, this test method will provide repeatable potentiostatic and potentiodynamic anodic polarization measurements that will reproduce data determined by others at other times and in other laboratories provided all laboratories are testing reference samples from the same lot of Type 430 stainless steel.
1.2 Values stated in SI units are to be regarded as the standard. Inch-pound units given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:G5–94 (Reapproved 2004)
Standard Reference Test Method for
Making Potentiostatic and Potentiodynamic Anodic
Polarization Measurements
This standard is issued under the fixed designation G5; the number immediately following the designation indicates the year of original
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2 Samples of a standard ferritic Type 430 stainless steel
(UNS S43000) used in obtaining standard reference plot are
1.1 This test method covers an experimental procedure for
available for those who wish to check their own test procedure
checking experimental technique and instrumentation. If fol-
and equipment.
lowed, this test method will provide repeatable potentiostatic
3.3 Standard potentiostatic and potentiodynamic polariza-
and potentiodynamic anodic polarization measurements that
tion plots are supplied with the purchase of the reference
will reproduce data determined by others at other times and in
material. These reference data are based on the results from
otherlaboratoriesprovidedalllaboratoriesaretestingreference
different laboratories that followed the standard procedure,
samples from the same lot of Type 430 stainless steel.
using that material in 1.0 N H SO . Maximum and minimum
2 4
1.2 Values stated in SI units are to be regarded as the
current values are shown at each potential to indicate the
standard. Inch-pound units given in parentheses are for infor-
acceptable range of values.
mation only.
3.4 Thistestmethodmaynotbeappropriateforpolarization
1.3 This standard does not purport to address all of the
testing of all materials or in all environments.
safety concerns, if any, associated with its use. It is the
3.5 This test method is intended for use in evaluating the
responsibility of the user of this standard to establish appro-
accuracy of a given electrochemical test apparatus, not for use
priate safety and health practices and determine the applica-
in evaluating materials performance. Therefore, the use of the
bility of regulatory limitations prior to use.
plots in Figs. 1 and 2 orAppendix X2 is not recommended to
2. Referenced Documents evaluate alloys other than Type 430, or lots of Type 430 other
than those available throughASTM.The use of the data in this
2.1 ASTM Standards:
test method in this manner is beyond the scope and intended
E1338 Guide for Identification of Metals and Alloys in
useofthistestmethod.Usersofthistestmethodareadvisedto
Computerized Material Property Databases
evaluate test results relative to the scatter bands corresponding
G3 Practice for ConventionsApplicable to Electrochemical
to the particular lot of Type 430 stainless steel that was tested.
Measurements in Corrosion Testing
G107 Guide for Formats for Collection and Compilation of
4. Apparatus
Corrosion Data for Metals for Computerized Database
4.1 The test cell should be constructed to allow the follow-
Input
ing items to be inserted into the solution chamber: the test
3. Significance and Use electrode, two auxiliary electrodes, a Luggin capillary with
salt-bridge connection to the reference electrode, inlet and
3.1 The availability of a standard procedure, standard ma-
outletforaninertgas,andathermometer.Thetestcellshallbe
terial, and a standard plot should make it easy for an investi-
constructed of materials that will not corrode, deteriorate, or
gator to check his techniques. This should lead to polarization
otherwise contaminate the test solution.
curves in the literature which can be compared with confi-
dence.
NOTE 1—Borosilicate glass and TFE-fluorocarbon have been found
suitable.
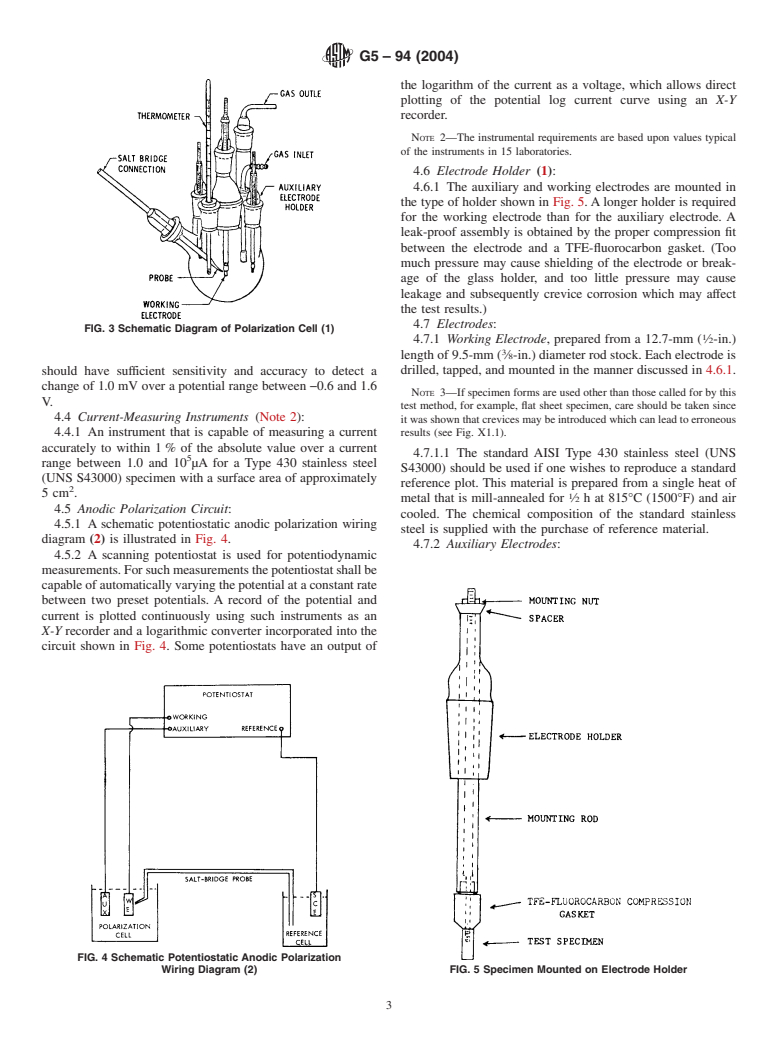
4.1.1 A suitable cell is shown in Fig. 3 (1). A 1-L,
This test method is under the jurisdiction of ASTM Committee G01 on roundbottomflaskhasbeenmodifiedbytheadditionofvarious
Corrosion of Metals and is the direct responsibility of G01.11 on Electrochemical
necks to permit the introduction of electrodes, gas inlet and
Measurements in Corrosion Testing.
Current edition approved Nov 1, 2004. Published November 1, 2004. Originally
´1
approved in 1969. Last previous edition approved in 1999 as G5–94(1999) . DOI:
10.1520/G0005-94R04. These standard samples are available from Metal Samples, P.O. Box 8,
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Mumford,AL36268. Generally, one sample can be repolished and reused for many
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM runs. This procedure is suggested to conserve the available material.
Standards volume information, refer to the standard’s Document Summary page on Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
the ASTM website. this test method.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
G5–94 (2004)
CURRENT DENSITY (µA/cm )
FIG. 1 Typical Standard Potentiostatic Anodic Polarization Plot
CURRENT DENSITY (µA/cm )
FIG. 2 Typical Standard Potentiodynamic Anodic Polarization Plot
outlet tubes, and a thermometer. The Luggin probe-salt bridge specimen supplied, the potentiostat should have a potential
separates the bulk solution from the saturated calomel refer- range from−0.6 to 1.6 V and an anodic current output range
enceelectrode,andtheprobetipcanbeeasilyadjustedtobring from 1.0 to 10 µA.
it in close proximity with the working electrode. 4.3 Potential-Measuring Instruments (Note 2):
4.2 Potentiostat (Note 2): 4.3.1 The potential-measuring circuit should have a high
11 14
4.2.1 Apotentiostatthatwillmaintainanelectrodepotential input impedance on the order of 10 to 10 V to minimize
within 1 mV of a preset value over a wide range of applied current drawn from the system during measurements. Such
currents should be used. For the type and size of standard circuits are provided with most potentiostats. Instruments
G5–94 (2004)
the logarithm of the current as a voltage, which allows direct
plotting of the potential log current curve using an X-Y
recorder.
NOTE 2—The instrumental requirements are based upon values typical
of the instruments in 15 laboratories.
4.6 Electrode Holder (1):
4.6.1 The auxiliary and working electrodes are mounted in
the type of holder shown in Fig. 5.Alonger holder is required
for the working electrode than for the auxiliary electrode. A
leak-proof assembly is obtained by the proper compression fit
between the electrode and a TFE-fluorocarbon gasket. (Too
much pressure may cause shielding of the electrode or break-
age of the glass holder, and too little pressure may cause
leakage and subsequently crevice corrosion which may affect
the test results.)
4.7 Electrodes:
FIG. 3 Schematic Diagram of Polarization Cell (1)
4.7.1 Working Electrode, prepared from a 12.7-mm ( ⁄2-in.)
lengthof9.5-mm( ⁄8-in.)diameterrodstock.Eachelectrodeis
drilled, tapped, and mounted in the manner discussed in 4.6.1.
should have sufficient sensitivity and accuracy to detect a
change of 1.0 mVover a potential range between−0.6 and 1.6
NOTE 3—If specimen forms are used other than those called for by this
V.
test method, for example, flat sheet specimen, care should be taken since
4.4 Current-Measuring Instruments (Note 2):
itwasshownthatcrevicesmaybeintroducedwhichcanleadtoerroneous
4.4.1 An instrument that is capable of measuring a current results (see Fig. X1.1).
accurately to within 1% of the absolute value over a current
4.7.1.1 The standard AISI Type 430 stainless steel (UNS
range between 1.0 and 10 µA for a Type 430 stainless steel
S43000) should be used if one wishes to reproduce a standard
(UNS S43000) specimen with a surface area of approximately
reference plot. This material is prepared from a single heat of
5cm .
metal that is mill-annealed for ⁄2 h at 815°C (1500°F) and air
4.5 Anodic Polarization Circuit:
cooled. The chemical composition of the standard stainless
4.5.1 A schematic potentiostatic anodic polarization wiring
steel is supplied with the purchase of reference material.
diagram (2) is illustrated in Fig. 4.
4.7.2 Auxiliary Electrodes:
4.5.2 A scanning potentiostat is used for potentiodynamic
measurements.Forsuchmeasurementsthepotentiostatshallbe
capableofautomaticallyvaryingthepotentialataconstantrate
between two preset potentials. A record of the potential and
current is plotted continuously using such instruments as an
X-Y recorder and a logarithmic converter incorporated into the
circuit shown in Fig. 4. Some potentiostats have an output of
FIG. 4 Schematic Potentiostatic Anodic Polarization
Wiring Diagram (2) FIG. 5 Specimen Mounted on Electrode Holder
G5–94 (2004)
4.7.2.1 Twoplatinumauxiliaryelectrodesarepreparedfrom oxygen-free gas such as hydrogen, argon, or nitrogen at a rate
high-purity rod stock. Each electrode is drilled, tapped, and of 150 cm /min for a minimum of ⁄2 h.
mounted with a TFE-fluorocarbon gasket in the same manner 5.5 Prepare the working electrode surface within1hofthe
as the working electrode. A large platinum sheet sealed into a experiment.Wetgrindwith240-gritSiCpaper,wetpolishwith
glass holder is also acceptable. 600-gritSiCpaperuntilpreviouscoarsescratchesareremoved,
4.7.2.2 A platinized surface may be utilized because of the rinse, and dry. (Drilled and tapped specimens can be threaded
increased surface area. This may be accomplished by cleaning onto an electrode holder rod and secured in a lathe or electric
the surface in hot aqua regia (3 parts concentrated HCl and 1 drill for this operation.)
part concentrated HNO ), washing, and then drying. Both 5.6 Determinethesurfaceareabymeasuringalldimensions
electrodes are platinized by immersing them in a solution of to the nearest 0.01 mm, subtracting the area under the gasket
3%platinicchlorideand0.02%leadacetateandelectrolyzing (usually 0.20 to 0.25 cm ).
at a current density of 40 to 50 mA/cm for4or5min (1, 3). 5.7 Mount the specimen on the electrode holder as de-
The polarity is reversed every minute. Occluded chloride is scribedin4.6.1.Tightentheassemblybyholdingtheupperend
removed by electrolyzing in a dilute (10%) sulfuric acid of the mounting rod in a vise or clamp while tightening the
solution for several minutes with a reversal in polarity every mounting nut until the gasket is properly compressed.
5.8 Degrease the specimen just prior to immersion and then
minute.Electrodesarerinsedthoroughlyandstoredindistilled
water until ready for use. Since certain ions can poison these rinse in distilled water.
5.9 Transfer the specimen to the test cell and adjust the
electrodes, periodic checks of platinized platinum potentials
against a known reference electrode should be made. salt-bridge probe tip so it is about 2 mm or 2 times the tip
diameter, whichever is larger from the specimen electrode.
4.7.2.3 Alternatively, graphite auxiliary electrodes can be
used, but material retained by the graphite may contaminate 5.10 Record the open-circuit specimen potential, that is, the
corrosion potential, after 55 min immersion. If platinum
subsequentexperiments.Thiscontaminationcanbeminimized
by using high-density graphite or avoided by routinely replac- counter electrodes and hydrogen gas are used, record the
platinum potential 50 min after immersion of the specimen.
ing the graphite electrode.
5.11 Potential Scan:
4.7.3 Reference Electrode (4):
5.11.1 Start the potential scan or step 1 h after specimen
4.7.3.1 Asaturated calomel electrode with a controlled rate
immersion, beginning at the corrosion potential (E ) for
of leakage (about 3 µL/h) is recommended. This type of
corr
potentiodynamic measurements and the nearest 50-mV incre-
electrode is durable, reliable, and commercially available.
ment above E for the potentiostatic measurements. Proceed
Precautions shall be taken to ensure that it is maintained in the
corr
through+1.60 V versus saturated calomel electrode (SCE)
propercondition.Thepotentialofthecalomelelectrodeshould
(active to noble).
be checked at periodic intervals to ensure the accuracy of the
5.11.2 In the potentiostatic method, use a potentiostatic
electrode. For other alloy-electrolyte combinations a different
potential step rate of 50 mVevery 5 min, recording the current
reference electrode may be preferred in order to avoid con-
at the end of each 5-min period at potential. These steps are
tamination of the reference electrode or the electrolyte.
repeated until a potential of+1.6 V SCE is reached.
4.7.3.2 Alternatively, a saturated calomel electrode utilizing
5.11.3 In the potentiodynamic method, use a potentiody-
a semi-permeable membrane or porous plug tip may be used.
namic potential sweep rate of 0.6 V/h (65%) recording the
These may require special care.
current continuously with change in potential from the corro-
sion potential to+1.6 V SCE.
5. Experimental Procedure
5.12 Plot anodic polarization data on semilogarithmic paper
5.1 Prepare 1 L of 1.0 N H SO fromA.C.S. reagent grade
2 4
in accordance with Practice G3, (potential-ordinate, current
acidanddistilledwater,forexample,byusing27.8mLof98%
density-abscissa).Ifapotentiostatwithalogarithmicconverter
H SO /Lof solution. Transfer 900 mLof solution to the clean
2 4
is used, this plot can be produced directly during the measure-
polarization cell.
ment.
5.2 Place the platinized auxiliary electrodes, salt-bridge
probe, and other components in the test cell and temporarily
6. Standard Reference Plots
close the center opening with a glass stopper. Fill the salt
6.1 Standard polarization plots prepared from data obtained
bridge with test solution.
by following the standard procedure discussed in this test
NOTE 4—When using a controlled leakage salt bridge, the levels of the
method are supplied with the purchase of reference material.
solutioninthereferenceandpolarizationcellsshouldbethesametoavoid
TypicaldataareshowninFig.1andFig.2 (5).Theplotsshow
siphoning. If this is impossible, a closed solution-wet (not greased)
a range of acceptable current density values at each potential.
stopcock can be used in the salt bridge to eliminate siphoning, or a
The average corrosion potential is−0.52 V, and the average
semi-permeable membrane or porous plug tip may be used on the salt
platinized platinum potential is−0.26 V.
bridge.
NOTE 5—The plots in Fig. 1 and Fig. 2 correspond to a lot ofType 430
5.3 Bring the temperature of the solution to 30 6 1°C by
stainless steel that is no longer available from ASTM (after July 1992).
immersing the test cell in a controlled-temperature water bath
Figs. 1 and 2 are presented primarily for the discussion of
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.