ASTM D6010-96(2006)
(Practice)Standard Practice for Closed Vessel Microwave Solvent Extraction of Organic Compounds from Solid Matrices
Standard Practice for Closed Vessel Microwave Solvent Extraction of Organic Compounds from Solid Matrices
SIGNIFICANCE AND USE
Extraction of organic pollutants from wastes can provide information on the susceptibility of compounds to leeching, water quality changes, or other site conditions.
Rapid heating, in combination with temperatures in excess of the atmospheric boiling point of organic solvents, reduces sample extraction times.
Small amounts of solvents (30 mL) are used resulting in reduced sample preparation cost and time.
SCOPE
1.1 This practice describes the closed vessel microwave extraction of soils, sediments, sludges, and wastes for subsequent determination of solvent extractable semivolatile and nonvolatile organic compounds by such techniques as gas chromatography and gas chromatography-mass spectrometry.
1.1.1 Compounds listed in Tables 1-5 can be extracted from the preceding materials.
1.2 This test method is applicable to samples that will pass through a 10-mesh (approximately 2-mm opening) screen.
1.3 The detection limit and linear concentration range for each compound is dependent on the gas chromatograph or gas chromatograph-mass spectrometer technique employed and may be found in the manual accompanying the instrument used.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. See Section for specific hazard statements.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D6010 − 96(Reapproved 2006)
Standard Practice for
Closed Vessel Microwave Solvent Extraction of Organic
Compounds from Solid Matrices
This standard is issued under the fixed designation D6010; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2.2 Other Standards:
United States Environmental Protection Agency (USEPA),
1.1 This practice describes the closed vessel microwave
Test Methods for Evaluating Solid Waste Volume
extraction of soils, sediments, sludges, and wastes for subse-
1A:Laboratory Manual Physical/Chemical Methods
quent determination of solvent extractable semivolatile and
Title21,CodeofFederalRegulations(CFR),Part1030,and
nonvolatile organic compounds by such techniques as gas
Title 47, Part 18
chromatography and gas chromatography-mass spectrometry.
1.1.1 CompoundslistedinTables1–5canbeextractedfrom
3. Summary of Practice
the preceding materials.
3.1 This procedure ensures intimate contact of the sample
1.2 This test method is applicable to samples that will pass
matrix with 115°C extraction solvent.
through a 10-mesh (approximately 2-mm opening) screen.
3.2 A1 to 5-g portion of a solid sample is extracted in a
1.3 The detection limit and linear concentration range for
sealed microwave transparent extraction vessel with 30 mL of
each compound is dependent on the gas chromatograph or gas
acetone-hexane (1+1).
chromatograph-mass spectrometer technique employed and
3.3 Up to 12 samples may be extracted simultaneously.
may be found in the manual accompanying the instrument
3.4 After extraction the vessels are cooled to room
used.
temperature, opened, and the solvent and sample are separated
1.4 This standard does not purport to address all of the
by decanting, filtration, or centrifuging.
safety concerns, if any, associated with its use. It is the
3.5 This practice provides a sample suitable for analysis by
responsibility of the user of this standard to establish appro-
gas chromatography or gas chromatography-mass spectrom-
priate safety and health practices and determine the applica-
etry.
bility of regulatory limitations prior to use. See Section 8 for
specific hazard statements.
4. Significance and Use
4.1 Extraction of organic pollutants from wastes can pro-
2. Referenced Documents
vide information on the susceptibility of compounds to
2.1 ASTM Standards:
leeching, water quality changes, or other site conditions.
D1193Specification for Reagent Water
4.2 Rapid heating, in combination with temperatures in
D3976Practice for Preparation of Sediment Samples for
excess of the atmospheric boiling point of organic solvents,
Chemical Analysis
reduces sample extraction times.
D5368TestMethodsforGravimetricDeterminationofTotal
Solvent Extractable Content (TSEC) of Solid Waste
4.3 Smallamountsofsolvents(30mL)areusedresultingin
Samples
reduced sample preparation cost and time.
5. Interferences
This practice is under the jurisdiction of ASTM Committee D34 on Waste
5.1 Methodinterferencesmaybecausedbycontaminantsin
Management and is the direct responsibility of Subcommittee D34.01.06 on
solvents, labware, and other hardware used in sample process-
Analytical Methods.
ing that lead to discrete artifacts or elevated baselines in gas
Current edition approved Feb. 1, 2006. Published March 2006. Originally
approved in 1996. Last previous edition approved in 2001 as D6010-96(2001). chromatograms. The analyst must demonstrate, through the
DOI: 10.1520/D6010-96R06.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from the Superintendent of Documents, U.S. Government Printing
the ASTM website. Office, Washington, DC 20402.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6010 − 96 (2006)
TABLE 1 Continued
analysisofreagentblanks,thatthesystemandthematerialsare
Spike Level, Average
free from interferents.
Analyte RSD, %
A
mg/kg Recovery, %
5.2 The use of high-purity solvents helps to minimize
3-Methylcholanthrene 5.0 117 8.6
interference problems.
Methyl methanesulfonate 5.0 48.5 28
2-Methylnaphthalene 5.0 104 9.3
5.3 Matrixinterferencesarecausedbycontaminantsthatare
2-Methylphenol 5.0 95.1 8.5
coextracted from the sample. The extent of matrix interfer-
4-Methylphenol 5.0 92.4 11
ences may vary considerably from sample to sample.
Naphthalene 5.0 95.0 12
1-Naphthylamine 5.0 57.8 8.7
5.4 Aftercleaning,vessellinersandcoversshouldbestored
2-Naphthylamine 5.0 73.5 9.0
in a clean environment to prevent accumulation of contami- 2-Nitroaniline 5.0 100 7.7
3-Nitroaniline 5.0 96.8 8.5
nants.
4-Nitroaniline 5.0 99.0 8.5
TABLE 1 Semivolatile Analyte Recovery from Freshly Spiked
Nitrobenzene 5.0 88.4 19
Topsoil
2-Nitrophenol 5.0 85.3 10
4-Nitrophenol 5.0 104 6.0
Spike Level, Average
Analyte RSD, %
A
mg/kg Recovery, % N-nitroso-di-n-butylamine 5.0 97.5 9.3
N-nitroso-di-n-propylamine 5.0 87.5 20
Acenaphthene 5.0 97.6 9.8 N-nitrosopiperidine 5.0 90.8 7.6
Acenaphthylene 5.0 100 10 Pentachlorobenzene 5.0 101 9.1
Acetophenone 5.0 92.2 12 Pentachloronitrobenzene 5.0 109 9.7
4-Aminobiphenyl 5.0 77.3 9.5 Pentachlorophenol 5.0 86.2 8.1
Aniline 5.0 68.1 7.5 Phenacetin 5.0 97.0 12
Anthracene 5.0 108 9.2 Phenanthrene 5.0 109 8.5
Phenol 5.0 97.3 9.2
Benzidine 5.0 0
Benzoic acid 5.0 42.3 13 2-Picoline 5.0 7.7 30
Pronamid 5.0 120 11
Benzo(a)anthracene 5.0 113 9.4
Benzo(b)fluoranthene 5.0 Pyrene 5.0 113 8.4
Benzo(k)fluoranthene 5.0 116 9.3 1,2,4,5-Tetrachlorobenzene 5.0 91.2 8.6
Benzo(g,h,i)perylene 5.0 111 4.7 2,3,4,6-Tetrachlorophenol 5.0 104 7.3
Benzo(a)pyrene 5.0 110 8.6 1,2,4-Trichlorobenzene 5.0 89.3 11
2,4,5-Trichlorophenol 5.0 95.1 12
Benzyl alcohol 5.0 96.1 9.0
Bis(2-chloroethoxy)methane 5.0 92.4 9.8 2,3,6-Trichlorophenol 5.0 96.4 6.7
2-Fluorobipenyl 2.5 92.9 8.0
Bis(2-chloroethyl)ether 5.0 96.0 11
Bis(2-chloroisopropyl)ether 5.0 95.2 12 2-Fluorophenol 5.0 95.4 7.7
Bis(2-ethylhexyl)phthalate 5.0 116 9.3 Nitrobenzene-d 2.5 92.2 9.8
4-Bromophenylphenyl ether 5.0 108 9.0 Phenol-d 5.0 98.9 9.7
Butyl benzyl phthalate 5.0 116 9.8 Terphenyl-d 2.5 112 10
4-Chloroaniline 5.0 97.0 9.2 2,4,6-Tribromophenol 5.0 92.3 7.7
1-Chloronaphthalene 5.0 104 12
A
The topsoil was dry when spiked. The number of determinations was three.
2-Chloronaphthalene 5.0 91.8 7.3
Determinations were made by gas chromatography-mass spectrometry.All recov-
4-Chloro-3-methylphenol 5.0 107 12
eries were corrected for analyte losses incurred during blowdown evaporation of
2-Chlorophenol 5.0 94.5 7.8
solvent to determine, specifically, recoveries by microwave extraction.
4-Chlorophenyl phenyl ether 5.0 106 9.7
B
Determined as azobenzene.
Chrysene 5.0 111 8.8
Dibenzo(a,j)acridine 5.0 10.6 34
6. Apparatus
Dibenzo(a,h)anthracene 5.0 110 5.9
Dibenzofuran 5.0 98.8 9.9
6.1 Microwave Heating System—A laboratory microwave
Di-n-butyl phthalate 5.0 113 9.4
heating system capable of delivering a minimum of 900 W of
1,2-Dichlorobenzene 5.0 89.9 12
1,3-Dichlorobenzene 5.0 87.6 13 microwave energy. The system should be capable of 1%
1,4-Dichlorobenzene 5.0 87.3 13
power adjustments and 1-s time adjustments. The microwave
3,3-Dichlorobenzidine 5.0 96.8 12
unit must be capable of measuring and controlling solvent
2,4-Dichlorophenol 5.0 97.5 8.0
2,6-Dichlorophenol 5.0 93.1 12
temperaturewithinanextractionvessel.Themicrowavecavity
Diethyl phthalate 5.0 111 8.0
should be constructed so as to prevent any possible metal to
Dimethylaminoazobenzene 5.0 116 11
metal arcing from occurring within the cavity.The oven cavity
7,12-Dimethylbenz(a)anthracene 5.0 128 7.0
αα-Dimethylphenethylamine 5.0 7.0 4.1 should be equipped with an exhaust ventilation sufficient to
2,4-Dimenthylphenol 5.0 107 9.4
provide ten chamber exchanges/min. The ventilation exhaust
Dimethyl phthalate 5.0 106 8.4
should contain an air flow sensor and a solvent sensor capable
4,6-Dinitro-2-methylphenol 5.0 57.6 9.3
2,4-Dinitrophenol 5.0 17.2 39 of detecting no air flow and solvent concentrations below their
2,4-Dinitrotoluene 5.0 98.2 6.2
lower explosive limits and shutting the microwave source off.
2,6-Dinitrotoluene 5.0 98.5 9.9
B The cavity shall have a 360° oscillating turntable to ensure
1,2-Diphenylhydrazine 5.0 108 11
Di-n-octyl phthalate 5.0 117 12
even sample heating and be capable of removing contained
Ethyl methanesulfonate 5.0 77.9 10
vessel-vented solvents. Safety interlocks to shut off magnetron
Fluoranthene 5.0 110 8.7
power output shall be contained in the cavity door opening
Fluorene 5.0 101 10
Hexachlorobenzene 5.0 108 8.9
mechanism. The system shall comply with the Department of
Hexachlorobutadiene 5.0 89.5 11
Health and Human Services Standards under the CFR, Part
Hexachlorocyclopentadiene 5.0 60.9 14
1030.10, Subpart (c)(1), (c)(2), and (c)(3), for microwave
Hexachloroethane 5.0 83.7 13
Indeno(1,2,3-cd)pyrene 5.0 99.2 6.2
leakage. The system should have Federal Communication
Isophorone 5.0 88.7 8.5
Commission (FCC) type approval for operations under FCC
D6010 − 96 (2006)
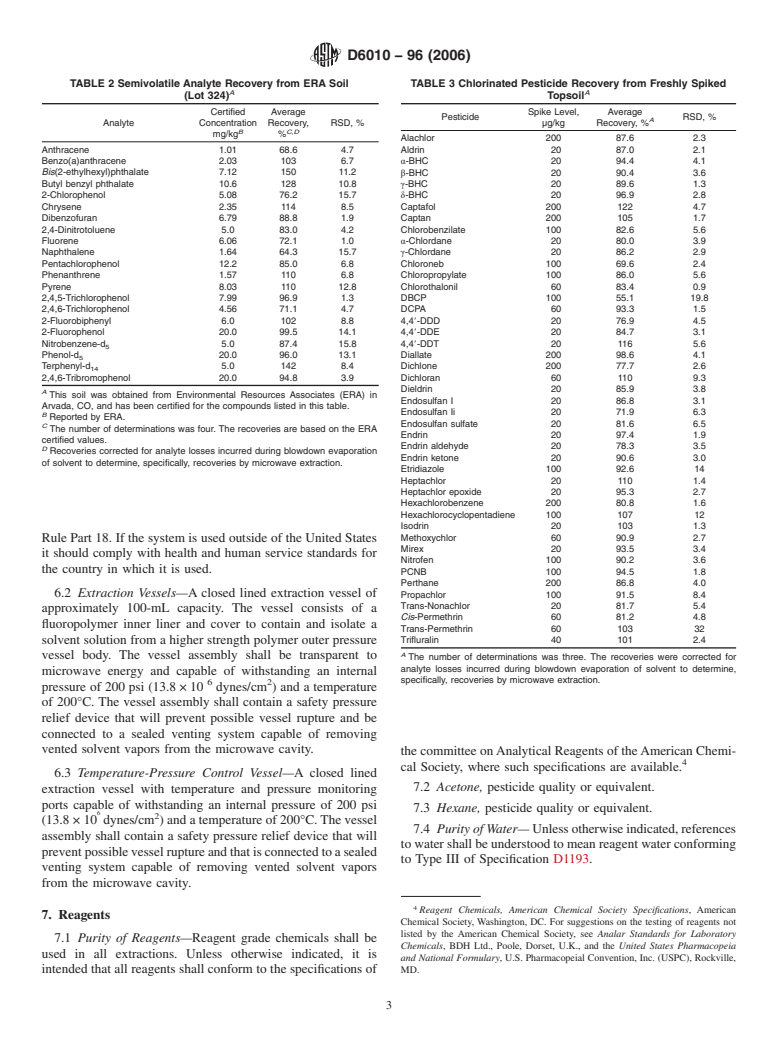
TABLE 2 Semivolatile Analyte Recovery from ERA Soil TABLE 3 Chlorinated Pesticide Recovery from Freshly Spiked
A A
(Lot 324) Topsoil
Certified Average Spike Level, Average
Pesticide RSD, %
A
Analyte Concentration Recovery, RSD, % µg/kg Recovery, %
B C,D
mg/kg %
Alachlor 200 87.6 2.3
Anthracene 1.01 68.6 4.7 Aldrin 20 87.0 2.1
Benzo(a)anthracene 2.03 103 6.7 α-BHC 20 94.4 4.1
Bis(2-ethylhexyl)phthalate 7.12 150 11.2
β-BHC 20 90.4 3.6
Butyl benzyl phthalate 10.6 128 10.8 γ-BHC 20 89.6 1.3
2-Chlorophenol 5.08 76.2 15.7 δ-BHC 20 96.9 2.8
Chrysene 2.35 114 8.5 Captafol 200 122 4.7
Dibenzofuran 6.79 88.8 1.9 Captan 200 105 1.7
2,4-Dinitrotoluene 5.0 83.0 4.2 Chlorobenzilate 100 82.6 5.6
Fluorene 6.06 72.1 1.0 α-Chlordane 20 80.0 3.9
Naphthalene 1.64 64.3 15.7 γ-Chlordane 20 86.2 2.9
Pentachlorophenol 12.2 85.0 6.8 Chloroneb 100 69.6 2.4
Phenanthrene 1.57 110 6.8
Chloropropylate 100 86.0 5.6
Pyrene 8.03 110 12.8 Chlorothalonil 60 83.4 0.9
2,4,5-Trichlorophenol 7.99 96.9 1.3 DBCP 100 55.1 19.8
2,4,6-Trichlorophenol 4.56 71.1 4.7 DCPA 60 93.3 1.5
2-Fluorobiphenyl 6.0 102 8.8 4,48-DDD 20 76.9 4.5
2-Fluorophenol 20.0 99.5 14.1 4,48-DDE 20 84.7 3.1
Nitrobenzene-d 5.0 87.4
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.